
How parasitic worms could be used against multiple sclerosis and other serious diseases
No one wants a parasitic worm infection and they can be bad for your health, but studies show diseases such as asthma, multiple sclerosis and Crohn’s disease can be reduced if your body is a host to these blood drinkers
By Jamie Morton
It’s a disgusting but true fact that humans have long lived with parasites that dwell within our bodies, feeding off us and sometimes causing sickness and disease.
Today, it’s estimated a billion people are infected by parasitic worm infections, mostly in developing nations.
Tapeworm in Chinese man’s brain four years removed by British scientists
Hookworms routinely infect humans through the skin – usually the foot – before migrating to the lungs and eventually establishing themselves in the gut where they can persist for years, feeding on blood. That can lead to iron and blood deficiency in the human host, affecting growth and development, as well as fatigue and general weakness.
But it wasn’t always just poorer countries affected by them – and it was only relatively recently that Western populations shed the worms they’d long carried.
“We were evolved to carry parasites all of the time – from birth to death,” explains Professor Graham Le Gros, a world-leading immunologist and the director of Wellington’s Malaghan Institute of Medical Research. “The simple introduction of sanitation and drugs that cleared the worms from our intestine stopped the reinfection cycle, and we essentially became worm free overnight.”
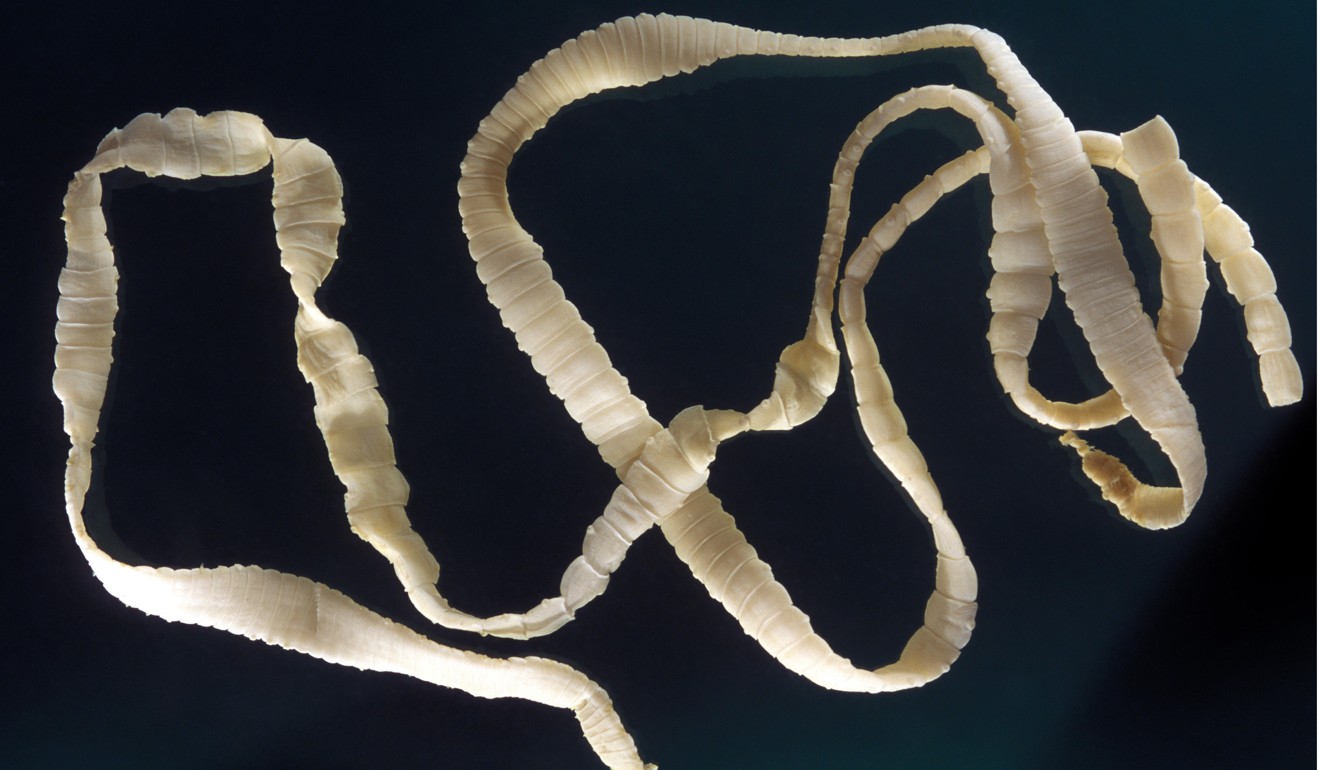
Some of these parasites are much more ghastly than others. The tapeworm can grow up to 15 metres long and live inside a human for three decades. But the hookworm, measuring just a few millimetres and estimated to affect 500 million people, is barely noticeable if in small enough numbers.
“If you have zero to 25 hookworms, you won’t feel a thing and you’ll be completely asymptomatic – but if you go from 25 to 50, you’ll start to have a bit of tummy grumbling,” Le Gros explains. “If you have 100 worms, you start to feel ill – and if you have 500, you’ll be looking seriously sick.”
Hookworms were once notorious for infecting coal miners, whose sallow skin had been initially thought to have been caused by coal dust, before it was revealed poor sanitation and the resulting parasites were in fact to blame.
Are colon-cleansing products necessary for a healthy gut?
In badly affected countries of the tropics and subtropics, hookworms are the leading causes of maternal and child disease, although cases are rarely fatal. In susceptible children, hookworms cause intellectual, cognitive and growth retardation, intrauterine growth retardation, prematurity, and low birthweight among newborns born to infected mothers.
In a newly published study, funded by the Health Research Council of New Zealand, Malaghan researchers have discovered a link between asthma and hookworm therapy that could open the door to potential new treatments.
While traditional thinking had been that blood feeding only occurred once the parasites entered the gut, postdoctoral researcher Tiffany Bouchery used a preclinical model to demonstrate that this actually began happening as soon it was inside the body.

“What we’ve found, quite unexpectedly, is that the worm starts blood feeding as soon as it enters the body, in the first three days of infection,” says Le Gros. “This insight could lead to new ways of targeting the species of hookworm that commonly infect humans, because once it is in the gut it’s very hard to deal with.”
Using this insight, the team used quinolones – antimalarial drugs – to inhibit the parasites’ ability to blood feed even before it reached the gut, preventing them from growing and persisting in the body.
The drug inhibits the iron-detoxification pathway which allows the worm to safely process iron in the blood. “While not what we set out to find, this piece of research is very exciting.”
Can eating parasitic worms or their eggs treat some diseases?
In terms of its potential and where it could lead, improved therapies for the billion people suffering from hookworm infection would be a major milestone in the fight against tropical diseases.
“It also gives us a deeper mechanistic insight into how other worms may be similarly affected when they first start migrating into the body and how we might use this knowledge to work with worms to our advantage.”
The findings suggested that further investigation of the iron-detoxification pathway could one day lead to the development of other drugs or vaccine targets against hookworm.
There are also interesting studies that show multiple sclerosis can be reduced if you have parasite infections. And when we talk about worms being good or bad, it depends how many you’ve got
Other research has even used hookworms themselves as an agent to fight pathogens and disease.
In one Australian study, 12 human participants were each experimentally infected with hookworm, and then given gradually increasing doses of gluten to reveal how the parasites could reduce the symptoms of coeliac disease.
“There are also interesting studies that show multiple sclerosis can be reduced if you have parasite infections,” says Le Gros.
“And when we talk about worms being good or bad, it depends how many you’ve got, obviously. But maybe they could teach us much more about how to use our immune systems.”
“But if we learn what the worms actually do, we could avoid the blunderbuss approach and maybe we could solve things like Crohn’s disease, by shooting down inflammatory conditions like the worms do.”
Le Gros said science was now on the cusp of exciting breakthroughs in the fight against disease, as knowledge of our immune system grows exponentially.
Our immune system is a complex system of structures and processes that has evolved to protect us from disease. Every day, our bodies are under attack from a wide variety of foreign and potentially infectious organisms, such as bacteria, viruses and parasites. Molecular and cellular components of our immune system travel around our bodies, patrolling for invading organisms and responding when necessary.

Our body has two main types of immune response – innate and adaptive. The innate immune system consists of cells and proteins that are present and ready to mobilise and fight microbes at the site of infection. Innate immunity has a number of set strategies for recognising and dealing with infections, without the need to be trained to recognise them.
The adaptive immune system is unable to respond instantly to infections, and needs time to adapt to recognise them.
Having learned to respond, it ias then able to remember particular germs that have infected the body previously so that when, or if, such infections try to infect the body again, the system is able to respond effectively with speed and precision.
Colony of 14 parasitic worms extracted from woman’s eye, in first human infestation
Immunology was the branch of biomedical science that dealt directly with it, and included research into the development of the immune system, as well as its daily functions and dysfunctions.
Le Gros called it the “future of human health”. “It’s game-changing,” he says. “As we better understand how our immune system works, and how it can be harnessed to fight disease, we’re poised for new, gentler and more effective treatments for cancer, asthma and allergy, multiple sclerosis, Alzheimer’s, gut disease and many other debilitating diseases.”
Vaccines and immunisations have already given a glimpse of the power of immunotherapy – teaching the immune system to fight disease by mimicking a natural infection.
“What is less well known, is that our immune system can also protect us from developing non-infectious diseases such as cancer. And research has shown that our immune system plays an important role in the development of allergic disorders and in many common disorders not traditionally viewed as immunologic, including metabolic and neurological diseases such as Alzheimer’s.”
Last year, the Malaghan Institute took the first step in developing New Zealand’s answer to the latest in cancer vaccines. CAR-T cell therapy was a revolutionary new approach to fighting cancer by redirecting a patient’s own immune cells to impart long-lasting protection against the disease.
“Later this year, through a collaboration with an international biotech group Hunan Zhaotai Medical Group, we’re aiming to conduct our first small CAR-T clinical trials, with the goal of developing a more effective and longer lasting way to fight certain cancers.”
Biotech boss backing gene therapy to solve ageing crisis seeks injection of urgency, but scientists preach patience
Understanding the relationship between the immune system, our food environment and the macro and microorganisms that live in and on us was also a big focus for the institute this year.
“By undertaking research into the basic biology of the immune system and developing immunotherapies and vaccines that supercharge the immune system, or regulate overactive immune responses, our scientists are striving to make a genuine difference to the way we treat disease.”

