Hong Kong government unveils plan to regulate use of medical devices in beauty clinics
Beauticians will be banned from operating high-risk devices while they can use other machines either on their own or under supervision of a doctor
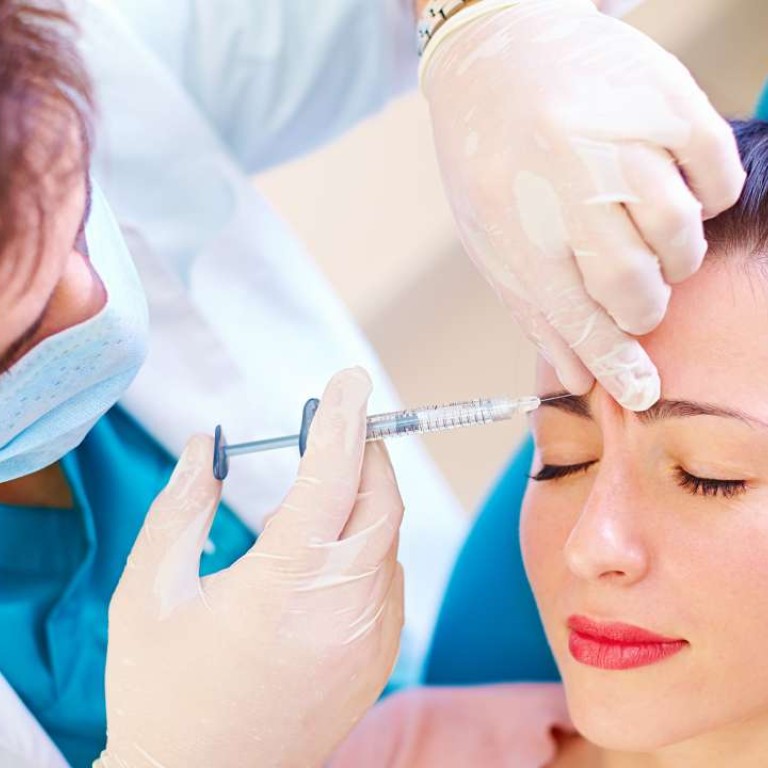
Beauticians will be banned from operating common beauty devices such as skin lasers without the supervision of a doctor under a new government proposal.
The Food and Health Bureau said in a paper submitted to lawmakers that they wanted to eliminate unscrupulous practices in the beauty sector by classifying medical devices into four categories according to risk.
Devices defined as high-risk can only be operated by a doctor or under a doctor’s supervision.They include devices providing popular beauty treatments such as skin lasers, robotic hair restoration and high-intensity focused ultrasound.
Representatives of the beauty industry said the proposal was unfair to them and warned it would affect their business.
“I am very disappointed with the proposal,” Federation of Beauty Industry chairman Nelson Yip Sai-hung said.
He said most beauty salons were small-scale operations and they might not have the resources to employ doctors.

Secretary for Food and Health Dr Ko Wing-man denied the government was targeting the beauty industry, saying they wanted to ensure the safely of the public.
“It aims to provide a regulatory framework for all medical devices,” Ko said.
There are currently no restrictions on the manufacture, import, sale and use of medical devices.
According to the proposal, the government wants to place 20 types of medical devices into four categories according to risk.
Machines considered to be low risk are classified as type 4. There is no restriction on the operation of these devices, which can be run by non-medical practitioners. They are used for short-wave hair removal, cryo-electrophoresism and electrical neuromuscular stimulation.
Users of type 3 devices must complete training related to the devices they will use.
Type 2 devices must either be operated by a doctor or supervised by one, while only doctors can operate type 1 machines.

