Chinese drug maker Shanghai Green Valley launching US trial of Alzheimer’s treatment as it seeks global legitimacy
- The US$600 million global phase three trial aims to sign up 2,046 people across China, US and Europe, with the first 600 expected to join in next six months
- The US FDA in April gave the nod to conduct large-scale trials on Green Valley’s treatment known as GV-971
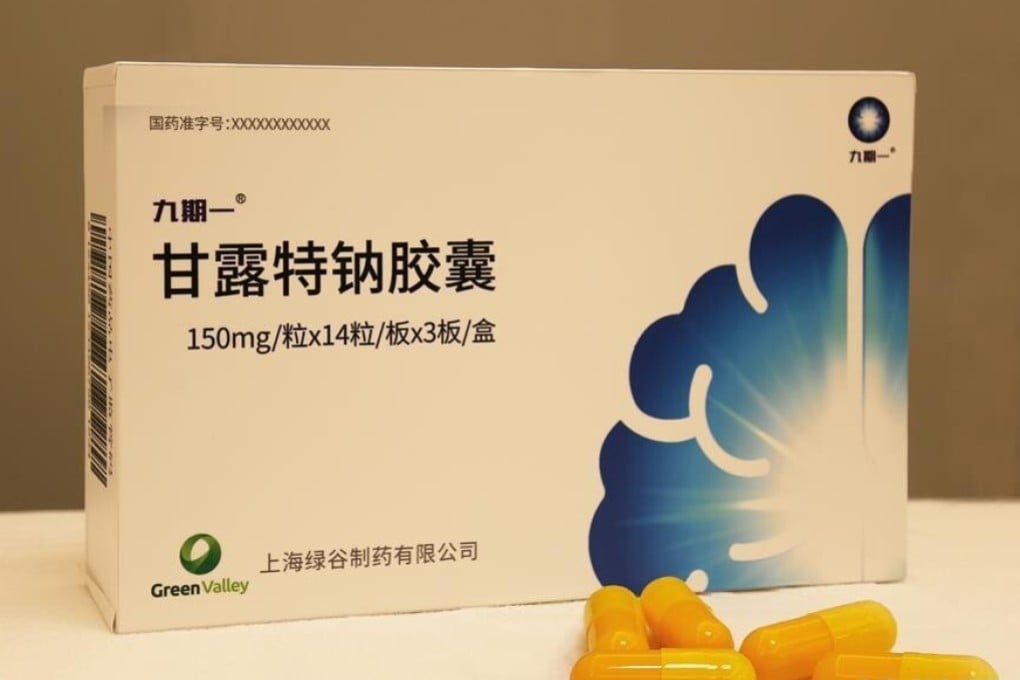
A new Chinese therapy for Alzheimer’s is embarking on a much-anticipated US study – the latest bid to revive hopes in the multibillion-dollar search for an effective medicine against the incurable disease.
The trial seeks to sign up 2,046 people across China, the US and Europe, with the first 600 expected to enroll in the next six months. Dosing of patients will begin in four weeks, according to study researchers in the US.

01:05
Grandma with Alzheimer's rescued unharmed after climbing out of high-rise building
With no medications currently able to change the course of the disorder, a successful drug could open a market worth as much as US$30 billion in the US alone, according to analysts at Sanford C. Bernstein. Pharmaceutical giants from AstraZeneca to Eli Lilly have spent billions of dollars over the years on more than 190 experimental drugs, only to see them fail one after another.