Coronavirus: vaccine roll-out for American children under 5 close after CDC panel vote
- US President Joe Biden’s administration plans to roll out the vaccines to the under-5 age groups as early as next week
- The 12-0 vote in favour of the move needs to be signed off by CDC director Rochelle Walensky for the government to start rolling out the vaccines
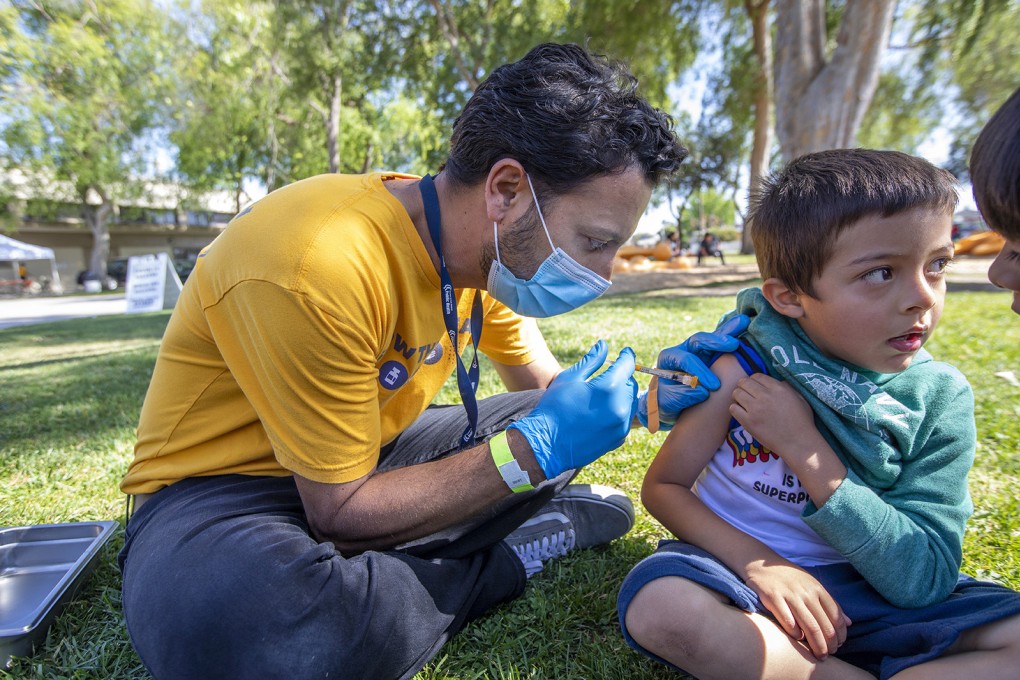
A panel of advisers to the US Centres for Disease Control and Prevention on Saturday voted to recommend Covid-19 vaccines for children as young as six months, making it likely a nationwide roll-out can start next week.
The 12-0 vote in favour of the move needs to be signed off by CDC Director Rochelle Walensky for the US government to start rolling out the vaccines for children aged 5 and under.
The US Food and Drug Administration on Friday authorised Moderna Inc’s shot for children aged six months to 5 years, and Pfizer-BioNTech’s vaccine for children aged six months to 4 years. Pfizer’s vaccine is already authorised for children over the age of 5.

“This infection kills children and we have an opportunity to prevent that,” Beth Bell, one of the doctors on the panel, said following the vote. “Here is an opportunity to prevent a known risk.”
President Joe Biden’s administration plans to roll out the vaccines to the under-5 age groups as early as next week.
“We will begin shipping millions of vaccine doses for kids to thousands of locations parents know and trust – including paediatricians’ offices, children’s hospitals, and pharmacies,” Biden said in a statement on Friday.
“As doses are delivered, parents will be able to start scheduling vaccinations for their youngest kids as early as next week, with appointments ramping up over the coming days and weeks.”