Bohr's atomic model 100 years old
Brilliant in its simplicity, Danish scientist's quantum leap in atomic theory has ever since provided valuable insights into chemical properties
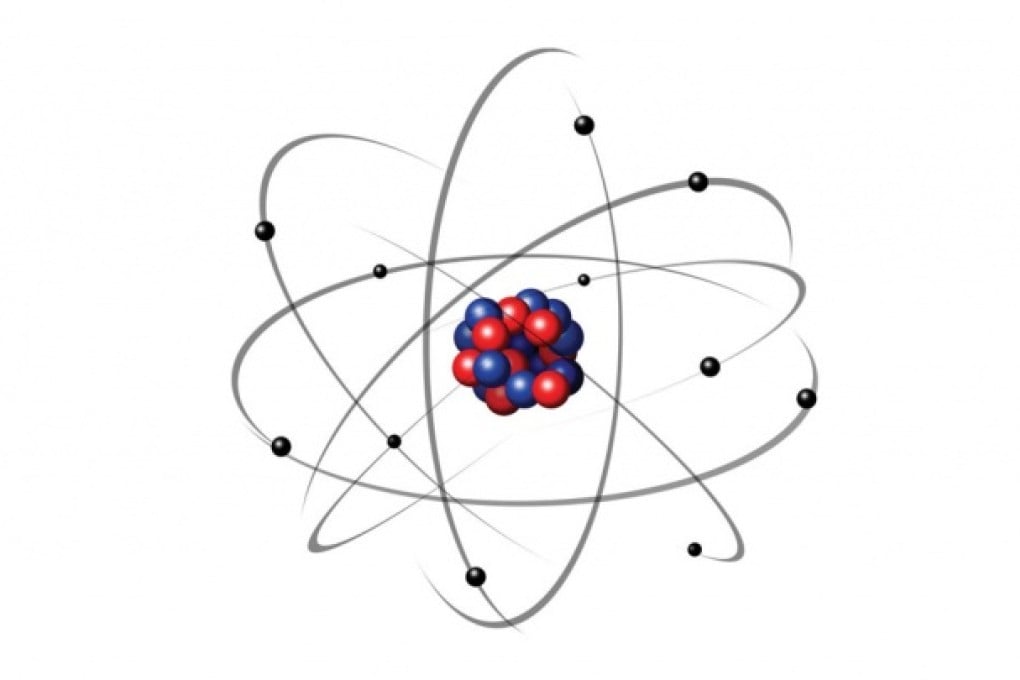
Picture an atom, and you may imagine spherical electrons orbiting a nucleus packed with particles like neutrons. Only certain orbits - quantum levels - are possible.
It's a simplistic model, yet provides insights into atoms and chemical properties, and this year marks 100 years since the model was first proposed by Danish physicist Niels Bohr.
The idea that matter comprises indivisible units dates back to Indian and Greek philosophers.
But it was not until around 1803 that the first useful atomic theory of matter was introduced, by British chemist-physicist John Dalton. He proposed that all matter is composed of atoms, which differ between elements and cannot be made or destroyed though can create compounds in chemical reactions.
Almost a century later, another Briton - physicist Joseph Thomson - discovered the electron through experiments with cathode rays. This led him to propose that atoms were made of electrons, or corpuscles as he called them, embedded in a sphere of uniform positive charge. His idea became known as the "plum-pudding model", with the electrons likened to plums within a popular pudding.
But just five years after Thomson published his model in 1904, it was disproved by an experiment directed by a New Zealand-born scientist who became known as the father of nuclear physics, Ernest Rutherford.