
- The mosquito-borne disease has claimed the lives of hundreds in the region, overwhelming health services
- Colonies of dengue-free mosquitoes are being introduced in high-risk areas, with promising results
A serious attack involves fever, sweating, vomiting, muscle spasms, driving thirst, thumping pain behind the eyes and in the joints. Weeks pass, tossing and turning in hellish delirium. And right now, a deadly wave of dengue fever is sweeping across Asia, killing hundreds and leaving tens of thousands sick, overwhelming health services and creating panic among politicians.
The Philippines has seen more than 146,000 confirmed cases of dengue so far this year, and at least 622 Filipinos have died from it. More than 49,000 cases have been reported in Thailand, with 64 deaths, and some 24,000 have been treated in Bangladesh, with at least 18 confirmed deaths.
The Aedes aegypti is a prolific breeder, but only the female mosquitoes feed on blood. They need the protein to produce eggs. Biting during the day, the Aedes aegypti rests out of sight in shady places, rarely travelling more than 100 metres from home base, preferring to live indoors, which means it is easily missed by insecticide fogging.

It can breed in the water left in an upturned bottle-cap, and it prefers to live in towns and cities, where food is abundant, whether in Asia, Australia, Africa, the lower parallels of the United States, or Central and South America. Think humid, think hot, chances are those places have it. (A sister species, Aedes albopictus, also carries dengue. It is dominant in Hong Kong and Guangzhou, and can survive at cooler temperatures than aegypti.)
The current Asian outbreak – Malaysia’s dengue numbers are now at an all-time high – is symptomatic of a larger plague spreading around the world, driven by rapid urbanisation in the “global south” combined with global warming. Current estimates put 40 per cent of the world’s population now at risk.
After decades of struggling to contain the disease with traditional methods such as vaccinations, it turns out that a microscopic bacterium, one already present in many species of insect, called Wolbachia, may hold the answer. And from universities to corporate headquarters, whether for public health or profit, the race to eradication is on.
Professor Scott O’Neill, 56, director of the non-profit World Mosquito Programme, is in charge of developing a sustainable, Wolbachia-based dengue-blocking method. A softly spoken entomologist and microbiologist with a shock of grey hair, O’Neill has studied and taught at universities in his native Australia and the US, including Yale.
As a PhD student at the University of Queensland, he studied the Wolbachia bacteria, microbes present in most insect species but, notably, not in the primary carrier of dengue, the black and white striped Aedes aegypti. During the course of his experiments with the bacteria, O’Neill began to wonder whether Wolbachia, inserted into Aedes aegypti mosquitoes, might stop them from transmitting diseases, and began working on a small research programme to find out.
Thirty years later, “it’s going well”, O’Neill says with a smile. His hunch had paid off: his evidence shows that Wolbachia slows a mosquito’s ability to replicate viruses like dengue, helps activate the host’s own immunity, and blocks viruses from being passed on to any offspring. “The data is looking better and better. The money is coming in. Now it’s a matter of delivering on the work.”

With funding in part from the Bill and Melinda Gates Foundation, O’Neill set up a formal research programme in 2005, using microscopic needles to inject Wolbachia into tens of thousands of mosquito embryos, manually, one at a time. Reaching adulthood, they were then released into netted enclosures to breed.
The team found the mosquitoes’ offspring also carried Wolbachia, and were resistant to dengue and other viruses. More crucially, here was a self-sustaining population of Aedes aegypti mosquitoes that could bite, and leave itchy welts, but were unable to transmit diseases to humans.
“It was a Eureka moment,” says O’Neill, of the realisation that this could eventually protect communities throughout the tropics from a range of diseases. “But you become used to not trusting results that seem too good.”
Most comfortable in the lab, O’Neill has had to adapt to running a sprawling global enterprise, dealing with government officials and scientists and billionaire philanthropists: the World Mosquito Programme is now a multimillion-dollar, multinational operation, with work under way in Australia, Brazil, Colombia, India, Indonesia, Vietnam, Mexico, Sri Lanka, New Caledonia, Kiribati, Fiji, Vanuatu and, most recently, Laos.

As the Wolbachia trial results roll in, communities around the world – attracted by the self-sustaining nature of the programme – are clamouring to take part. How to prioritise them?
“It’s a combination of a few things but mostly where the need is,” says O’Neill. “We’re really interested in cities that have large at-risk populations, so there’s a large potential impact from our work there […] Thailand is a huge dengue hot spot in southeast Asia. We’re very interested in it, we just don’t have the capacity right now to be there.”
The whole of Africa, too, is waiting.
A trial in Yogyakarta, Indonesia, is going so well – earlier data showed an 80 per cent reduction in dengue – that three more Indonesian cities will be next. At the same time, the Brazilian health ministry has funded expansion from Rio de Janeiro to a further three Brazilian cities.
It’s not always smooth sailing. It can be difficult, for example, to convince locals who have battled mosquitoes for most of their lives that it’s a good idea to release more of the bloodsuckers into their neighbourhoods.
Dengue fever is now like the flu, WHO chief says
Two years of consulting took place before the Yogyakarta trial, in Kronggahan II subdistrict. Most residents welcomed workers arriving to release the mosquitoes, but a few hundred wanted to know why the Australians were treating them like kelinci – guinea pigs – even though the programme was being run in partnership with one of Indonesia’s most respected universities, Gadjah Mada, and it had the blessing of not only city leaders, but the Sultan of Yogyakarta himself.
To prove the method’s efficacy, billionaire philanthropist Bill Gates allowed himself to be bitten in a Yogyakarta lab before the insects were shipped out for use in the trial.
Despite the urgency of combating mosquito-borne disease, some scientists remain sceptical of introducing foreign bacteria into common species, believing mosquitoes will evolve to the point where Wolbachia will no longer keep them from infecting humans.
O’Neill has conducted independent risk analyses of Wolbachia introduction, and Australia’s Commonwealth Scientific and Industrial Research Organisation has considered the possible ramifications of introducing the bacteria into mosquitoes. They concluded the risk of environmental consequence was negligible.
Evolution will definitely prevail. [Wolbachia] will work for perhaps a period of time, but whether it will work on a large scale – meaning, will that strain be able to survive in other parts of the world – we do not knowIvan Hung Fan-ngai, professor, University of Hong Kong
“Evolution will definitely prevail,” says Professor Ivan Hung Fan-ngai, from the University of Hong Kong.“[Wolbachia] will work for perhaps a period of time, but whether it will work on a large scale – meaning, will that strain be able to survive in other parts of the world – we do not know.”
The World Mosquito Programme has one biological trick working in its favour. Occurring naturally in nearly all Wolbachia strains, “Cytoplasmic incompatibility” means that when a Wolbachia-carrying male mosquito mates with a non-Wolbachia carrying female, the eggs will die. Wolbachia mosquitoes will, given time, outbreed all others.
The Wolbachia mosquitoes released in north Queensland over a 10-week period nine years ago are the ancestors of a Wolbachia-carrying population that is still there, still breeding, and still failing to transmit disease.
A victory for the programme, yes, but these were smaller communities. What about the world’s teeming, steamy metropolises packed with millions of vulnerable people?

In 2014, the World Mosquito Programme’s first citywide trial began, in the Australian community of Townsville, population about 187,000, in northern Queensland. “Mozzie Box” kits containing dried Wolbachia mosquito eggs – which can remain viable for months – were distributed to residents and schoolchildren for the four-year experiment. Just add water to the Mozzie Box and soon out float the Wolbachia-carrying mosquitoes.
The trial results, released last year, showed there hadn’t been any cases of locally transmitted dengue since Wolbachia mosquito populations were established in the city’s release areas.
The Townsville research also proved the Wolbachia method could be deployed on a citywide scale for a reasonable US$10 per person. The World Mosquito Programme’s target is to bring that down to about US$1 in bigger Asian cities, where dengue can take on plague proportions.
Cases of infection by Aedes albopictus, the Asian tiger mosquito that also carries dengue, have fallen 80 per cent at two small island sites in Guangzhou, thanks to a combination of the Wolbachia method and an alternative that uses radiation to sterilise male mosquitoes in the lab, before releasing them to mate with a local population of females, who can then produce no offspring, wiping out an entire generation.
All the people doing suppression are working with, or have their own, commercial company. As a for-profit it sort of makes sense [though] the goal might be less about public health and more about revenue streamsScott O’Neill, World Mosquito Programme
Xi Zhiyong, who collaborated with the World Mosquito Programme as recently as 2014, and is now director of the Sun Yat-sen University-Michigan State University Joint Centre of Vector Control for Tropical Diseases, ran the trials on Shazai and Dadaosha islands, in the Pearl River Delta, south of Guangzhou city, the area with the highest number of dengue cases in China. His system, which has been trialled before, would require a regular replenishment of Wolbachia mosquitoes to keep the wild population under control.
While initially expensive, the project’s costs would come down as the technology advanced, reports the Associated Press, estimating between US$42 and US$66 per acre of land per year. That’s roughly on par with current costs for agricultural pest sterilisation, and cheaper than some insecticide regimes.
O’Neill says Xi’s Guangzhou trial used methods developed by the World Mosquito Programme, but Xi’s approach is to eradicate the entire mosquito population, which, while effective for a time, will only ever be temporary.
“Once you stop, you get pretty fast rebounds, so it requires ongoing releases,” says O’Neill. “It’s a good business model, because someone has to keep buying new mosquitoes from him […] He has a commercial company […] All the people doing suppression are working with, or have their own, commercial company. As a for-profit it sort of makes sense [though] the goal might be less about public health and more about revenue streams.”
Why dengue fever prevention calls for attention to the big picture
US commercial firms such as Verily Life Sciences (an offshoot of Google’s parent company formerly known as Google Life Sciences) and Kentucky-based MosquitoMate use Wolbachia to suppress mosquito populations in the same way as one would carry out other cosmetic yard work, such as fertilising your lawn or chlorinating your pool.
“MosquitoMate starts releasing male ZAP mosquitoes weekly in your yard at the beginning of the mosquito season,” the company website reads. “Fact: male mosquitoes don’t bite. We release ZAP males to mate with Asian Tiger mosquito females, which are invasive to the USA. The resulting eggs do not hatch, decreasing your biting mosquito population. Our technology only impacts mosquitoes and does not harm or affect other insects, including bees and butterflies. It’s that simple.”
British biotech company Oxitec takes yet another approach, writing on its website that its copyrighted “Friendly™ Mosquitoes carry a ‘self-limiting’ gene, which means that when Friendly™ Mosquitoes mate with wild females, their offspring inherit a copy of this gene, which prevents them from surviving to adulthood. Since the offspring do not mature to reproduce, there is a reduction in the wild pest population.”
With few other options available, families, cities and communities that can afford it will pay to rid themselves of the pests that not only bring irritating itchy bites but also possibly debilitating, even fatal disease. The problem: far too large a number of those affected cannot.

One long-used solution for mosquito-plagued communities has been insecticides, which can beat back mosquito infestations for a time. But the insects soon roar back – South America had wiped out nearly all of its Aedes aegypti mosquitoes some decades ago, but after a period they repopulated almost the entire continent. Meanwhile, the poisons can damage other ecosystems and species, including humans.
Joseph Kam Kai-man, an associate professor at the Stanley Ho Centre for Emerging Infectious Diseases, at the Chinese University of Hong Kong, says, “The mosquitoes themselves, and our own human behaviour in terms of how we work and how we urbanise our cities, those are very significant factors. We don’t take care of the waters around us and so on.”
Kam says much of the knowledge and skills used in early mosquito-control programmes has been lost. There are distinct stages to a mosquito’s life cycle, he points out – from egg to larva to pupa to adult – and different methods are required for each. “People read scientific papers and push what they want,” says Kam. “I’ve seen so many mistakes by mosquito-control programmes – they don’t really know what they’re doing. They spray whatever they want into stagnant water and hope for the best.”
Like Hung, Kam is concerned the Wolbachia method will not be a long-term solution and, at some point, wild, non-Wolbachia-carrying mosquitoes will again take over. “I must congratulate the Australians on a very successful exercise in a small place, and people learn from success stories, but I think they should also learn from their failures,” he says.

Confident of success, Sanofi began producing the vaccine in 2013, before the release of final trial results, and financial analysts suggested the vaccine’s sales might be worth US$1 billion a year.
There are four known serotypes (or species variations) of dengue (DEN 1 to 4) and each is capable of causing the disease. A vaccine must prompt an immune response to all four types. Although recovery from one type of dengue provides lifelong immunity against that particular serotype, it also opens the way for a “severe dengue” infection from another serotype. So if someone has had dengue, it could be far worse the second or third time around.
The first large-scale Dengvaxia trial found that to provide full protection, the vaccine required three doses over 12 months, a regime that many saw as challenging. Perhaps most disappointing, Dengvaxia had an overall efficacy rate of only 56 per cent.
At the time, Alain Bernal, then Sanofi’s vice-president of global communications, said it was “important to remember that there is currently no vaccine available at all, so these findings remain a major public health advance”.
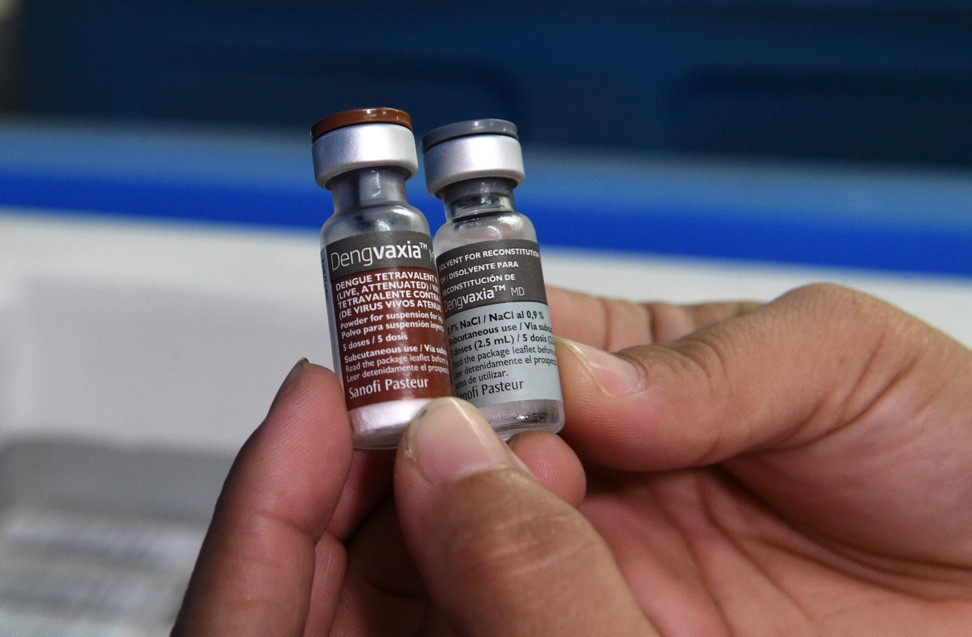
Despite initial problems, Sanofi began production in a new French facility, to great fanfare, and the Philippines government decided to be the first Asian nation to approve commercial sales, starting in 2016, with a Dengvaxia vaccination programme in schools.
The following year, Sanofi announced that those who had been vaccinated with Dengvaxia, but had not been infected previously, could be more at risk than others of a severe bout of the disease.
The Philippines’ health department suspended the programme, but the controversy continues to dog Sanofi, and there are lawsuits in the offing. The World Health Organisation now warns that Dengvaxia “should not be administered to people who have not previously been infected with dengue virus” and says that nations that still want to use Dengvaxia should consider pre-vaccination screening to ensure recipients have already had dengue.
HKU’s Hung says, “New vaccines – there are several currently on phase 2 trials – may be much more effective in terms of serotype 2 and [may] not have this kind of problem […] There’s always a way of speeding up the licensing of new vaccines if they have been proven to work very well.”
O’Neill, meanwhile, understands the “evolution” arguments against the long-term efficacy of the Wolbachia method. “As a biologist I know the system will evolve over time and likely become less effective – just as antibiotics have become less effective. The thing we don’t know is over what time-frame this will happen. Our current understanding suggests it won’t be quickly, so I think we can assume decades at least. I would be ecstatic if we got hundreds of years, but I won’t be around to see it.”
