Coronavirus: Sinovac shot causes ‘drastic drop’ in deaths, infections among Indonesian health workers; World Economic Forum to go ahead with Singapore meeting
- Indonesia found that Sinovac protected 100 per cent of 25,374 health workers from death, 96 per cent from hospitalisation and 94 per cent from infection
- Meanwhile, the World Economic Forum plans to go ahead with its annual meeting in Singapore this August despite a jump in coronavirus cases in the city state
Indonesia tracked 25,374 health workers in capital city Jakarta for 28 days after they received their second dose and found that the vaccine protected 100 per cent of them from death and 96 per cent from hospitalisation as soon as seven days after, said Health Minister Budi Gunadi Sadikin in an interview on Tuesday. The workers were tracked until late February.
Sadikin also said that 94 per cent of the workers had been protected against infection – an extraordinary result that goes beyond what was measured in the shot’s numerous clinical trials – though it is unclear if the workers were uniformly screened to detect asymptomatic carriers.
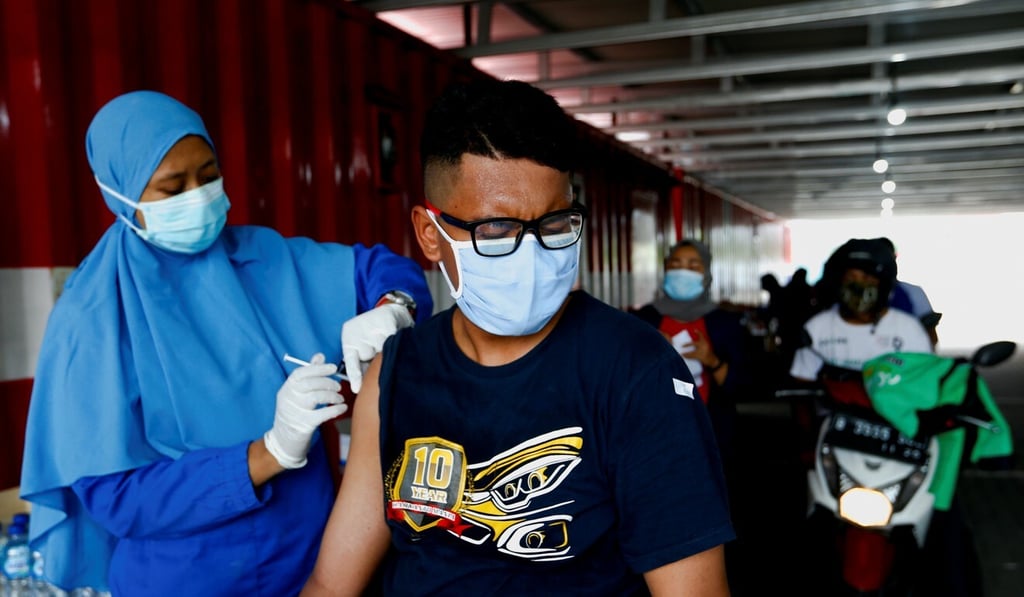
“We see a very, very drastic drop,” in hospitalisation and deaths among medical workers, Sadikin said. It is not known what strain of the coronavirus Sinovac’s shot worked against in Indonesia, but the country has not flagged any major outbreaks driven by variants of concern.