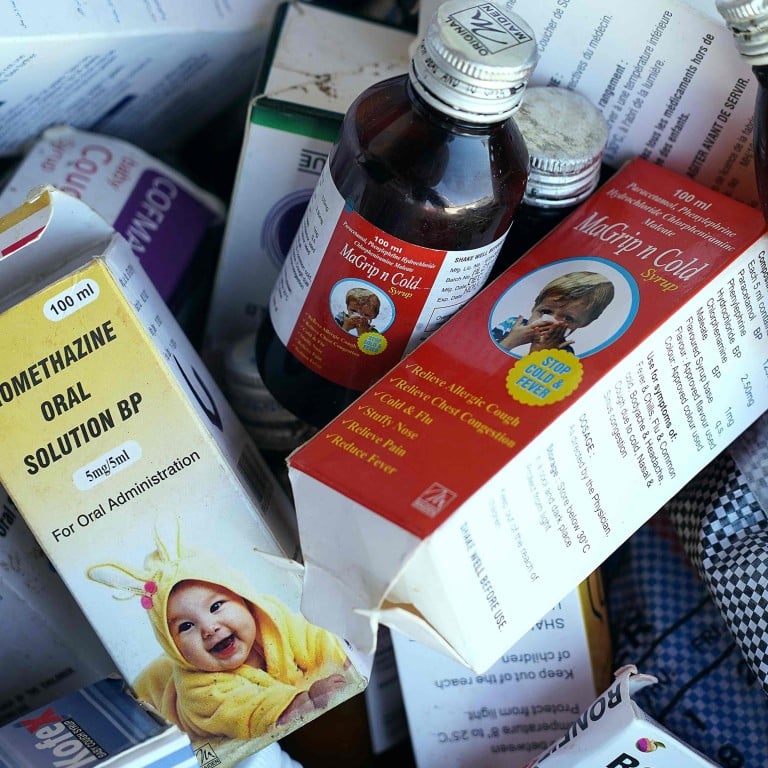
India halts cough syrup production at factory linked to Gambia deaths
- Production of cough syrup was ordered to stop after authorities inspected a Maiden Pharmaceuticals factory in Haryana state and found 12 violations
- WHO said last week lab tests at Maiden found ‘unacceptable’ amounts of diethylene glycol and ethylene glycol, which can be toxic
The health minister in Haryana state, Anil Vij, told Asia News International (ANI) that authorities inspected a Maiden factory near the town of Sonipat in the state and found 12 violations of good practices. Production was ordered stopped, Vij said.
The WHO said last week that laboratory analysis of four Maiden products – Promethazine Oral Solution, Kofexmalin Baby Cough Syrup, Makoff Baby Cough Syrup and Magrip N Cold Syrup – had “unacceptable” amounts of diethylene glycol and ethylene glycol, which can be toxic and lead to acute kidney injury.
India hopes ‘Pharma City’ will break China’s grip on industry
It is one of the worst such incidents involving drugs from India, often dubbed a “pharmacy of the world”.
News website Moneycontrol earlier quoted the Haryana drugs controller as saying in a report that Maiden did not perform quality testing of propylene glycol, diethylene glycol and ethylene glycol, while certain batches of propylene glycol did not have the manufacturing and expiry dates.
Diethylene glycol and ethylene glycol are used in antifreeze and brake fluids and other industrial applications but also as a cheaper alternative in some pharmaceutical products to glycerine, a solvent or thickening agent in many cough syrups.
Maiden executive Naresh Kumar Goyal declined to comment. He told Reuters last week that the company was trying to find out from its buyer what had happened in Gambia.

Maiden says on its website it has an annual production capacity of 2.2 million syrup bottles, 600 million capsules, 18 million injections, 300,000 ointment tubes and 1.2 billion tablets at three factories.
It said it sells its products at home and exports to countries in Asia, Africa and Latin America.
The cough syrups had been approved for export only to Gambia, India says, although the WHO says they may have gone elsewhere through informal markets.
India’s health ministry said last week that samples of all four Maiden products that had been exported to Gambia had been sent for testing to a federal laboratory and the results would “guide the further course of action as well as bring clarity on the inputs received/to be received from WHO.”
Health ministry officials and the WHO did not respond to requests for comment.

Indonesia also investigating
Indonesia will investigate cases of acute kidney injury which has caused the deaths of more than 20 children in its capital Jakarta this year, health authorities said on Wednesday.
Indonesia will coordinate with investigators from the WHO.
Indonesia’s drug regulator (BPOM) said in a statement the syrups were not registered in the country.
Its health ministry said it is talking to experts from the WHO that are investigating the case in Gambia and it has formed a team with the country’s paediatric association (IDAI) and a Jakarta-based hospital to look into the cases.
It said the illness had infected 40 children across the country so far but didn’t say when the cases were first reported.
IDAI was quoted by Indonesian newspaper Kompas as saying 131 cases have been reported between January and September, adding the cases in Gambia are unrelated to those in Indonesia.
The health ministry said early findings point to potential intoxication as a cause of the illness, but no definitive cause has been found yet. The ministry said further research was needed.

