
China’s Sinovac defends Brazil results but regulators to look hard at data
- Chinese firm seeks to clarify Brazilian partner’s announcement that CoronaVac is 50 per cent effective
- Company will double production capacity in February to meet overseas and domestic demand, chairman says
The low efficacy rate for Sinovac Biotech’s CoronaVac vaccine was announced on Tuesday by the Butantan Institute in Brazil, raising questions over the effectiveness of the shots.
But in Beijing on Wednesday, Sinovac Biotech chairman Yin Weidong insisted that the data showed CoronaVac was effective and safe.
“This [Brazilian] data shows that the vaccines have good efficacy and safety in all the phase 3 clinical trials,” Yin said. “We have accelerated the ramping up of production capacity.”
02:04
Indonesia says CoronaVac is 65.3% effective as Chinese-made Covid-19 vaccine makes inroads in Asia
It was the first public response from the company since the institute announced the 50.38 per cent efficacy rate – barely passing the threshold set by most health regulators and well below the 70 per cent recommended by the Chinese government and the World Health Organization.
After the disclosure, various regulators said they would assess the data carefully to decide whether to go ahead with buying or distributing the vaccine.
“If we are not satisfied with the safety and efficacy, we will not go through with the procurement,” Malaysian Science Minister Khairy Jamaluddin said on Twitter. Malaysia is still in talks with Sinovac for orders.
Regulators in Singapore and Hong Kong also said they would look at the data before rolling out the shots, though they have already placed orders.
Yin said Sinovac would proceed to apply for conditional approval from the Chinese regulator. It was given emergency use approval in mainland China several months ago and at least 7 million mainland Chinese have received the jabs.
Yin said a factory was already making 500 million doses a year, and a second one with the same capacity was expected to start production next month.
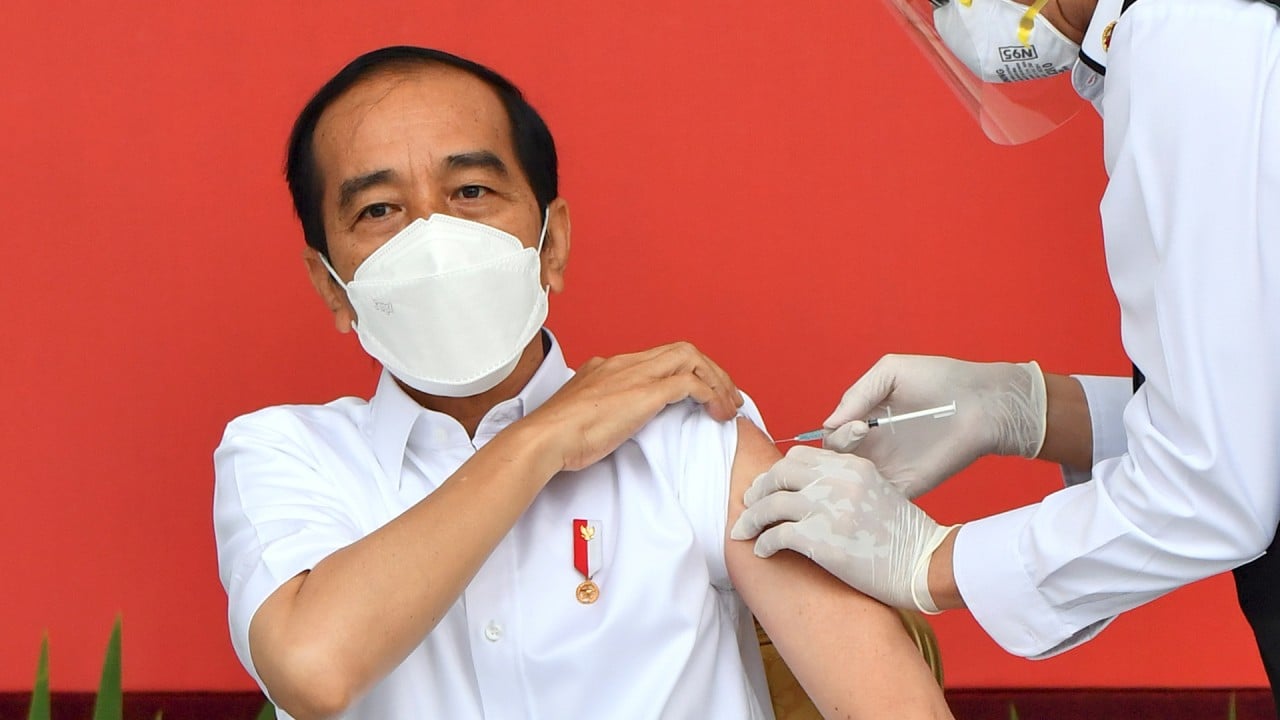
“However, these facilities are still far short of the orders we have received from many countries, including Indonesia, Malaysia, Singapore and the Philippines,” he said. “You can see that Indonesian President Joko Widodo received the vaccine today.”
02:24
Joko Widodo gets first Sinovac vaccine shot as Indonesia starts mass Covid-19 inoculations
The latest disclosure from the Butantan Institute follows a string of confusing announcements and delays since December, prompting calls in the scientific community for transparency.
Yin said CoronaVac was 100 per cent effective in preventing severe cases, 78 per cent effective for cases requiring treatment, and 50 per cent effective in “protecting medical workers”.
He also cited figures released previously by Turkey and Indonesia, saying the former showed an efficacy rate of 91 per cent and the latter 65 per cent.
“Generally speaking, all phase 3 clinical trial research data is of great significance for applying for conditional approval,” Yin said. “[The application] needs to follow the relevant protocol, to analyse data and have it reviewed. We will file for a market launch with conditions after the data reaches the standard for submission.”
Australian scientists raise doubts over whether AstraZeneca coronavirus vaccine can deliver herd immunity
Scientists said that in reviewing applications, regulators would examine what the company called the general efficacy rate, which is 50.4 per cent in the trials in Brazil. They would also look at the detailed data from Turkey, Indonesia and Chile.
Peter Smith, a professor at the London School of Hygiene and Tropical Medicine, said the main focus for regulators was symptomatic cases.
“Up to now the primary end point used for assessing efficacy has been based on all symptomatic cases (whether or not they required treatment) – i.e. their 50.4 per cent estimate,” Smith said.
Jerome Kim, director general of the International Vaccine Institute in Seoul, said: “The WHO lists 50 per cent as the lower bound. It meets the threshold for approval, but is not ideal.”
Vaccines by Pfizer and Moderna have achieved a much higher efficacy rate of around 95 per cent. A vaccine by Oxford-AstraZeneca has an efficacy rate of 70 per cent.
Smith said: “To what extent thinking will change in the light of other vaccines showing much higher efficacy is hard to assess, but the general criteria is that the benefits exceed the risks, which this [CoronaVac] vaccine would appear to satisfy,”
“Note that the efficacy of the AstraZeneca vaccine was substantially less than that of Pfizer (and Moderna) but to date only the UK ... and India has authorised the AstraZeneca vaccine.”
Hong Kong fourth wave: minister promises tough safety checks after Sinovac’s Covid-19 vaccine performs worse than expected in new study
He said the vaccine still met the minimum efficacy requirement set by regulators but they might look at other options. Vaccines by Pfizer and Moderna have achieved a much higher efficacy rate of around 95 per cent. A vaccine by Oxford-AstraZeneca has an efficacy rate of 70 per cent.
“To what extent thinking will change in the light of other vaccines showing much higher efficacy is hard to assess, but the general criteria is that the benefits exceed the risks, which this [CoronaVac] vaccine would appear to satisfy,” he said.
“Note that the efficacy of the AstraZeneca vaccine was substantially less than that of Pfizer (and Moderna) but to date only the UK ... and India has authorised the AstraZeneca vaccine.”

02:29
Brazil study shows China’s Sinovac vaccine less effective than earlier data on the Covid-19 shots
Tao Lina, a Shanghai-based vaccine expert, also said that the general efficacy of 50 per cent was acceptable.
“It is so far the most thorough disclosure of the efficacy data on a Chinese vaccine and it is the best way to address the challenges,” he said.
Yin did not say whether the company would release overall efficacy rates combining those in Brazil, Turkey and Indonesia.
Yin and the institute defended the lower efficacy rate in Brazil, saying it was the result of higher exposure to the coronavirus because the participants were all medical workers.
But Smith said it was unclear how that made a difference.
“The UK trial of the AstraZeneca vaccine was also among health workers. Without knowing exactly how they ascertained cases, it is difficult to comment on the Butantan argument,” he said.
If a Covid-19 vaccine passes human trials, how does it get approved?
Kim said Sinovac’s presentation of the data gave the impression that it was trying to hide the “bad data” and it would undermine the credibility of the vaccine.
“The confusion creates the impression that the company is not in control of the studies, the analysis, and the communications plan. When you release the data, you want the case to be as strong as possible that the data is correct, the analysis is correct and as strong and cogent as possible,” he said.
“This creates the unfortunate impression that presenting a strong, scientific case for safety, efficacy and quality is secondary to selling their vaccine. The Sinovac vaccine could be an important contribution to global health but fumbling its debut does not create confidence,” he said.
The Sinovac vaccine is one of the three China has approved for emergency use and is part of the national effort under way to inoculate priority groups, totalling as many as 50 million people.
Additional reporting by Ji Siqi


