Advertisement
Coronavirus: Chinese customs stalls vaccine shipment for trials in Canada
- Canadian research council says CanSino injections have not been approved to be sent from China
- Council says it is still working with Tianjin company and trials will start when the shots are delivered
Reading Time:3 minutes
Why you can trust SCMP
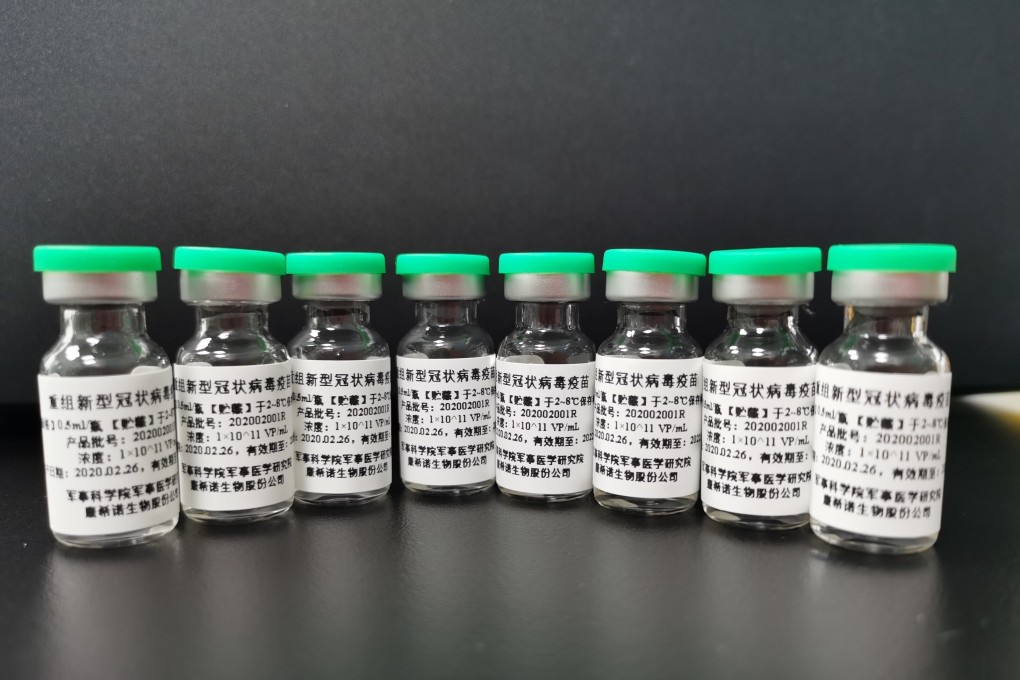
Clinical trials in Canada of an early Chinese front runner in the race for a coronavirus vaccine are in limbo, with Chinese customs authorities holding up shipment of the injections for weeks.
Health Canada gave approval for clinical trials for Ad5-nCoV, a potential vaccine developed jointly by Tianjin-based CanSino Biologics and Chinese military scientists, on May 16.
The scientist in charge of the trials, Dalhousie University professor Scott Halperin, said in mid-May that he expected the first of the three phases of trials to start later that month. But Halperin said three weeks ago that he had yet to receive the vaccines for the trials.
Advertisement
Health Canada said the vaccines were held up by China’s General Administration of Customs.
On Thursday, the National Research Council of Canada (NRC) confirmed to the South China Morning Post that the customs administration had still not allowed the shipment to proceed.
Advertisement
Advertisement
Select Voice
Choose your listening speed
Get through articles 2x faster
1.25x
250 WPM
Slow
Average
Fast
1.25x