Scientists say some coronavirus vaccines under trial need screening for raising HIV risk
- Researchers have raised concern about candidates made with a cold virus known as adenovirus 5
- It was found to increase the risk of HIV infection among certain men in trials over a decade ago
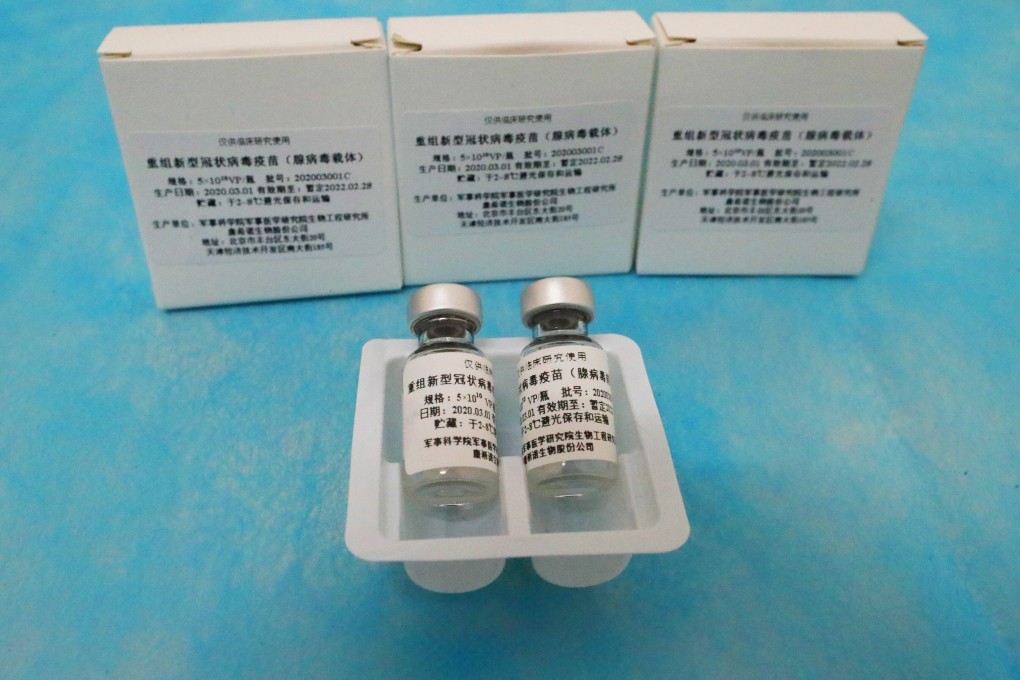
Some vaccines use what is known as a viral vector, or a modified virus that delivers the vaccine’s genetic material into the body’s cells. One vector, a cold virus known as adenovirus 5 (Ad5), is being used in two Covid-19 vaccines in advanced trials – one by Chinese company CanSino Biologics and another by Russia’s Gamaleya Research Institute.
But four scientists who ran international trials of an HIV vaccine candidate using Ad5 over a decade ago found it increased the risk of HIV infection for certain men. The researchers are concerned the same risks remain.
“Both the HIV and Covid-19 pandemics disproportionately affect vulnerable populations globally,” the researchers wrote in a letter published in The Lancet scientific journal this week. “Roll-out of an effective Sars-CoV-2 vaccine globally could be given to populations at risk of HIV infection, which could potentially increase their risk of HIV-1 acquisition.”
This “important safety consideration” should be thoroughly evaluated before further development of Ad5 vaccines for Sars-CoV-2, wrote the researchers, who all work for institutions in the United States, including the National Institutes of Health.

01:08
China promises its Covid-19 vaccines will be available worldwide at a ‘fair and reasonable’ price
It comes as some 10 experimental Covid-19 vaccines, developed using a range of technologies, have advanced into large final phase 3 trials, raising expectations that a vaccine to fight the pandemic is near after it has killed more than 1.1 million people and upended economies and livelihoods for billions.