
How China’s protectionist vaccine policy has backfired on Beijing
Strict screening process including Chinese clinical trials has left big foreign drug makers with a tiny share of the lucrative market
China’s latest public health scare has raised fresh questions over the government’s protectionist measures against foreign imports of vaccines.
Confidence in products made by Chinese pharmaceutical firms sank to a new low after a major producer was found selling large quantities of faulty vaccines that were supposed to protect infants against diphtheria, whooping cough and tetanus, and forging data for its rabies shots.
But Beijing’s strict vetting system means Chinese looking for alternatives will find it hard to track down vaccines on the mainland made by foreign companies, including products proven to be effective that are in wide use overseas. That policy has led to a growing number of mainland Chinese parents taking their children to Hong Kong and to other countries such as Japan for inoculations.
Health care booking agents cash in on China’s vaccine scandal amid rush to secure jabs at Hong Kong clinics
Economist Wang Fuzhong has called for the government to open the door wider to imported vaccines in the wake of the scandal, writing on his blog that this would activate market competition and eventually help to restore trust in domestic pharmaceuticals.
Wang said a competitive and efficient market would go a long way to improving safety in the industry, but a closed market had led to inefficiency, corruption and health risks.
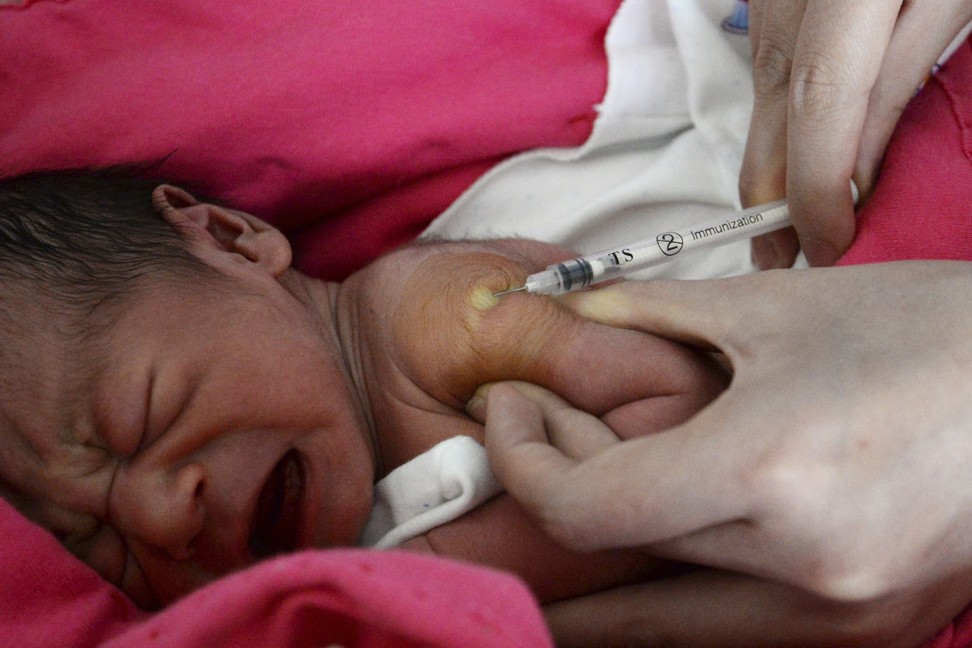
China puts all imported vaccines through a strict screening process, something UBS Securities pharmaceuticals analyst Zhao Bing said was needed given the importance of immunisation for public health.
One requirement is that all imported vaccines must go through Chinese clinical trials, but the review process can take years. In one typical case, the US Food and Drug Administration approved the first human papillomavirus (HPV) vaccine, an effective prevention against cervical cancer, in 2006. But it took a decade for the China Food and Drug Administration (CFDA) to approve the first HPV vaccine in the country. Mainland Chinese who did not want to wait instead went over the border to Hong Kong to receive the potentially life-saving jabs.
The already tiny share of imported vaccines in the Chinese market has gone down from 5 per cent in 2012 to 2.5 per cent last year, according to the National Institutes for Food and Drug Control (NIFDC), the agency in charge of vaccines for the CFDA.
Of the vaccines available in China, nearly 80 per cent are category 1 products that are included in the mandatory government immunisation programme, and they are almost exclusively supplied by Chinese companies. The remaining 20 per cent, or about 150 million vaccines, are category 2 products that are open to competition from foreign pharmaceuticals.
The CFDA also said Beijing would “suspend imports or registration” of inferior products if it found any.
Vaccine makers are given a licence for products approved for sale in China that must be renewed every five years, and Beijing can decide not to renew it without giving a reason.
That happened to Prevenar 7, a flagship Pfizer vaccine that protects infants from pneumonia, in 2015, when the CFDA refused to renew the import licence with no explanation. It took 18 months for the company to get approval to sell an upgraded version of the vaccine, Prevenar 13, in China. That left mainland Chinese with no access to Prevenar products for that period, while a doctor who smuggled some into the country was jailed.
George Kuo, chairman of privately-run Shanghai American-Sino Obstetrics and Gynaecology Service, illegally obtained 13,000 doses of 11 types of vaccines – including Prevenar 7 and 13 – from Singapore in that period for the clinic’s Chinese patients. Kuo was convicted of selling “fake drugs” by a Shanghai court and sentenced to seven years in jail, according to a Southern Weekend report. Under Chinese law, any medicine imported without a licence is regarded as “fake”.
Fake data – the disease afflicting China’s vaccine system
Foreign pharmaceutical firms are expected to meet standards for some vaccines that are higher or different from the international standards, making it more difficult for them to apply for licences.
Hong Kong-based business consultant Yolanda Yin said foreign firms often came up against the problem of different standards.
“It’s a hard choice for multinational companies – if they have to comply with Chinese standards, they may need to change their production techniques that comply with other countries’ standards,” Yin said. “Chinese players find it easier because they’re only operating in the domestic market.”
When China raised standards for vaccines in 2010, for instance, pharmaceutical companies like Sanofi Pasteur gradually retreated from the rabies immunisation sector in the country. Sanofi Pasteur didn’t immediately reply to requests seeking comment.
Roberto Bruzzone, Co-Director at HKU-Pasteur Research Pole at School of Public Health of the University of Hong Kong, said the Sanofi Pasteur vaccine “is used in many countries and has never been suspected of being sub-standard; hence it would seem logical to make it available also to the Chinese market”.
Chinese companies meanwhile thrived under the system, monopolising the lucrative rabies vaccine market. Changsheng Bio-tech became the second biggest rabies vaccine supplier in the country last year, with a 23 per cent market share, before it was found to have committed serious fraud in its production. Its rabies vaccine licence had been renewed by the CFDA in April, just three months before the data forgery was exposed.

Last year, the NIFDC said its tests had found that Sanofi Pasteur’s Pentacel, which protects against five diseases and is popular in China, was not effective enough. The agency found that the tetanus component in eight batches of the vaccine was substandard and suspended imports of the product, resulting in shortages across the country. Sanofi withdrew other batches of the vaccine awaiting testing in China, according to the NIFDC.
In April, three months before the faulty vaccines were revealed, the NIFDC said the Sanofi case showed the screening system “is highly sensitive and can reveal quality issues … and protect people’s health”.
Chinese drug regulators step up nationwide inspections after latest vaccine scandal
A former regional manager at Merck’s subsidiary in China, MSD, said vaccines supplied by Chinese pharmaceutical firms were still given preference by the provincial bodies that bought them for the government health programme.
“China supports its own drug companies and encourages people to buy medicines that are made in the country,” the former manager, who declined to be named, said.
Between them, GlaxoSmithKline (GSK), Merck, Sanofi and Pfizer controlled about 90 per cent of the vaccine market worldwide last year, according to London-based research firm EvaluatePharma. But it is a different story in China.
Just a month ago, state media outlets, including the official Xinhua news agency, were putting out reports saying it was “unnecessary to blindly believe in imported vaccines” because the Chinese-made products had “no obvious difference to imported ones in terms of quality standard, safety and use”.
After the latest revelations, the authorities will have a hard time convincing anxious parents. Many are now trying to find a way to access foreign vaccines, and over the border in Hong Kong – where all vaccines for children are imported brands – many private clinics say they are fully booked for immunisations for the next two months.

