
Release coronavirus vaccine efficacy data, ex-China drug watchdog chief urges country’s pharma firms
- Bi Jingquan urges Chinese pharmaceutical firms to be more transparent to build public trust in their products
- Shots by Sinovac, Sinopharm and CanSino Biologics have been approved for use in some countries without publishing comprehensive phase 3 clinical trial results
On the sidelines of the first day of the annual session of China’s top advisory body, Bi Jingquan said pharmaceutical firms should release efficacy information to build public trust.
“The companies should themselves know that, with data, the more transparency, the better,” Bi, the director of the China Food and Drug Administration between 2015 and 2018, said on Thursday.
“Publish all your data; it’s good for strengthening everybody’s trust.”

03:05
China-made coronavirus vaccines widely distributed despite efficacy concerns
Sinovac published combined phase 1 and 2 results for its CoronaVac vaccine in The Lancet medical journal in November but so far the company has not released efficacy data for the phase 3 trials through a peer-reviewed journal. Instead, governments in each of the countries where the trials took place have released their own data.
“China’s vaccines are not bad. The Centre for Drug Evaluation will in time release its evaluation report to the public,” said Bi, deputy director of the economic affairs committee of the Chinese People’s Political Consultative Conference.
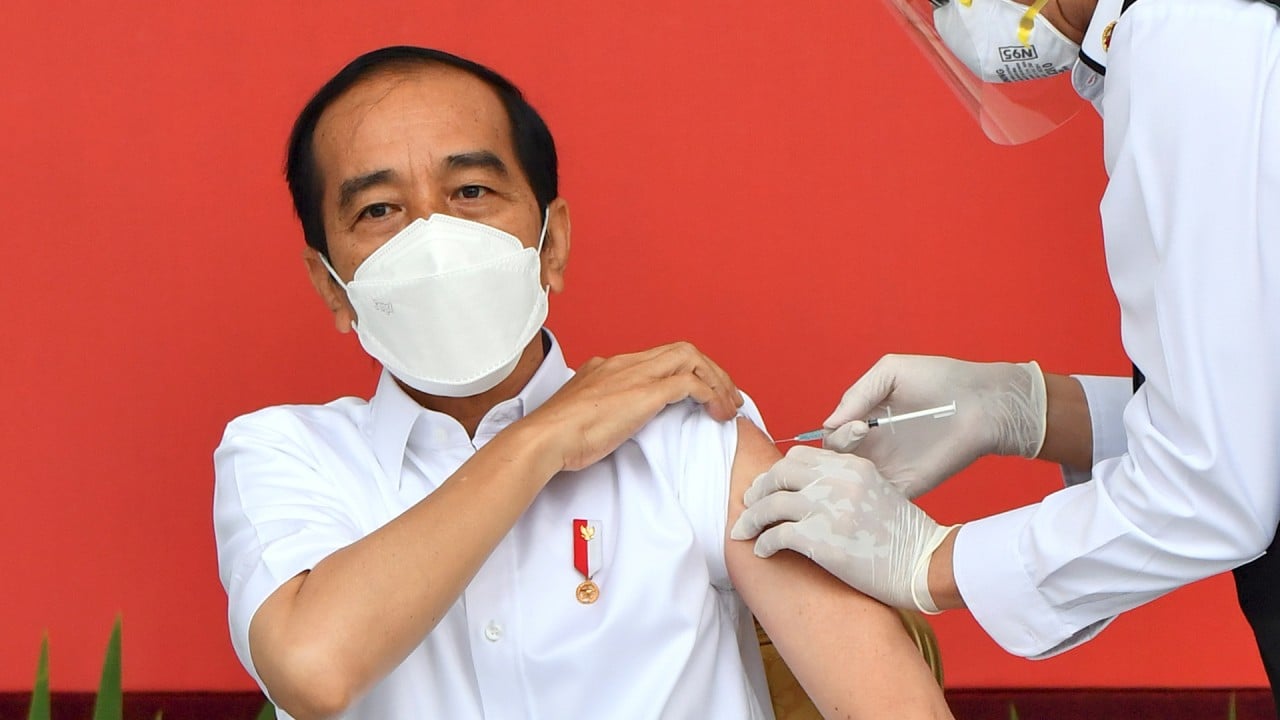
02:24
Joko Widodo gets first Sinovac vaccine shot as Indonesia starts mass Covid-19 inoculations
The Butantan Institute in Brazil, one of the CoronaVac’s phase 3 test sites, said in December that Sinovac had demanded it withhold the full result of the vaccine’s efficacy.


