Taiwan Covid-19 vaccines may be too late for US emergency approval
- Former health official flags competition problems for home-grown shots as global brands complete phase 3 clinical trials
- Two locally produced vaccines have finished phase 2 trials and are expected to be marketed on the island in the next few months
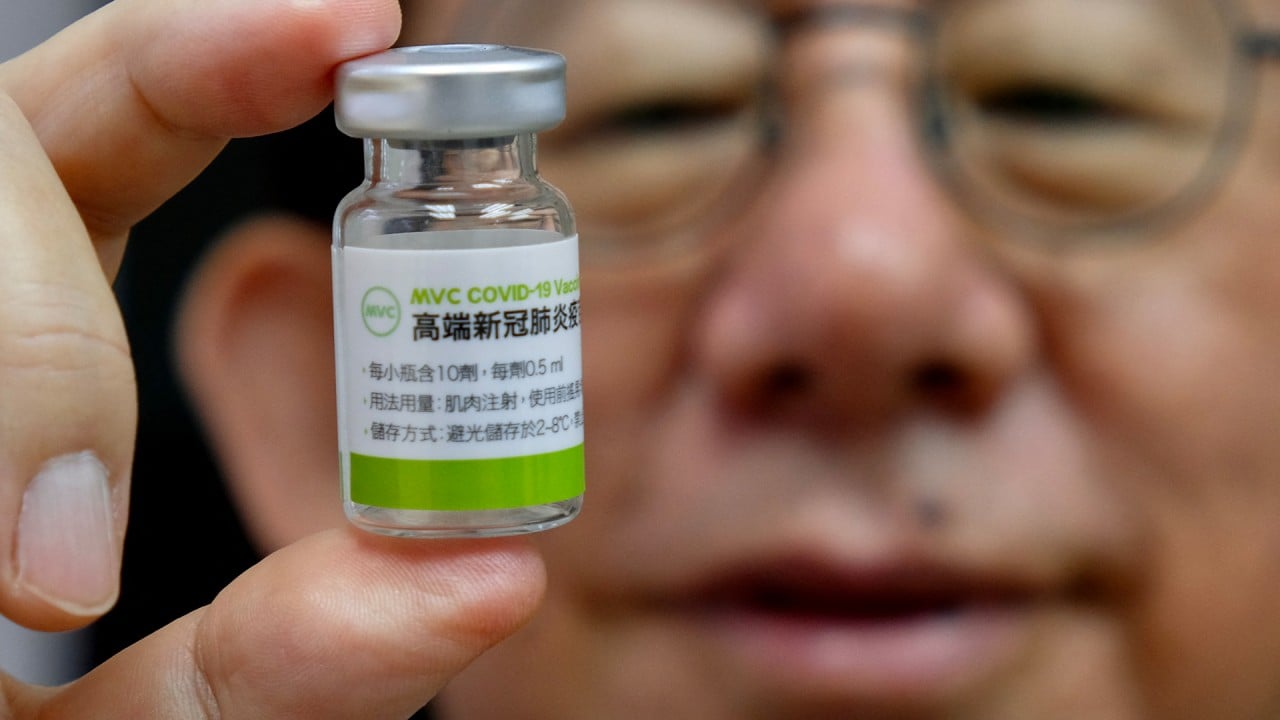
Taiwan’s Central Epidemic Command Centre has ordered 10 million doses of vaccines from two local developers – Medigen Vaccine Biologics and United Biomedical – with an option of buying 10 million more amid a recent Covid-19 outbreak that saw 135 new infections and eight deaths on Tuesday.
Coronavirus: Taiwan outbreak ‘appears to stabilise’ as new cases fall
Both firms have completed phase 2 clinical trials of their vaccines and are seeking local regulators’ EUA approval to market their shots, hopefully in the next few months, according to officials from the two companies.
But their vaccines could end up being marketed only in Taiwan, as the US FDA has suggested it may no longer issue EUA for new vaccine candidates, according to Su Ih-jen, former director general of the Centres for Disease Control.
“The outbreak in the US has stabilised and the vaccines developed by American companies are in abundance, meaning the US FDA does not have the need to approve EUA for vaccine candidates from other countries,” he said.
Su said a halt on EUA approvals by the US FDA would also make it difficult for locally developed vaccines to enter the global market, especially as a number of international brand products had already completed their third and final clinical trials and were ready to get full approvals for use in their home countries.
“But the local vaccines have only completed phase 2 clinical trials, and still have a long way to undergo the phase 3 trials,” Su said. He added that if fully approved global brand products entered the Taiwan market, the local brands would face stiff competition, with people more likely to opt for the foreign vaccines.
According to Reuters, Novavax has had discussions with regulators and said it does not expect to seek regulatory authorisation for its Covid-19 shot in the US, Britain and Europe until the third quarter of 2021.
Canadian developer Medicago said it was in discussions with the FDA for an EUA for its plant-based Covid-19 vaccine candidate, which is enhanced by a GlaxoSmithKline treatment, Reuters reported.
AstraZeneca from Britain also has discussed plans for its Covid-19 vaccine with US officials. However, The Wall Street Journal earlier this month reported it was considering skipping US emergency-use authorisation in favour of the more time-intensive application for a fully-fledged licence to sell the shot.
On Tuesday, Medigen said it was seeking international cooperation in phase 3 clinical trials of the company’s protein-based subunit vaccine – a type of vaccine whose purified pieces have been selected for their ability to stimulate immune cells – with the aim of getting permits for global marketing.
“[Medigen has signed a] memorandum of understanding with the National Institute of Hygiene and Epidemiology under the Ministry of Health in Vietnam and National University of Asuncion in Paraguay for cooperation in the clinical trials of our Covid-19 vaccine,” it said in a statement.
The company was also negotiating with Southeast Asian nations over Covid-19 vaccine development and business cooperation projects, Medigen said without naming the countries.
A company spokesman said cooperation with the Vietnamese institute was aimed at supplying vaccine to Vietnam, which has a population of over 95 million.
United Biomedical was not immediately available for comment.
Beijing repeats offer of Covid-19 vaccines as Taiwan reports 287 new cases
Local media reported that through its affiliated company in the US, United Biomedical had registered for phase 2 and 3 clinical trials in the US, but was yet to learn whether it could still apply for EUA from the US FDA.
They said that from early 2021 the company had pursued mid- and late-stage trials for its vaccine candidate in India and Brazil.
Taiwanese Health Minister Chen Shih-chung said local developers could still gain international approval through the World Health Organization’s immune-bridging study or validation of phase 3 clinical trials.