Advertisement
Coronavirus: Chinese regulator says vaccines must have 50 per cent efficacy, give 6 months’ immunity
- Products that meet the efficacy standard but have not completed their testing may also be used in emergencies, Chinese Centre for Drug Evaluation says
- Four Chinese vaccine candidates are undergoing clinical trials in the United Arab Emirates, Brazil, Saudi Arabia and Indonesia
Reading Time:2 minutes
Why you can trust SCMP
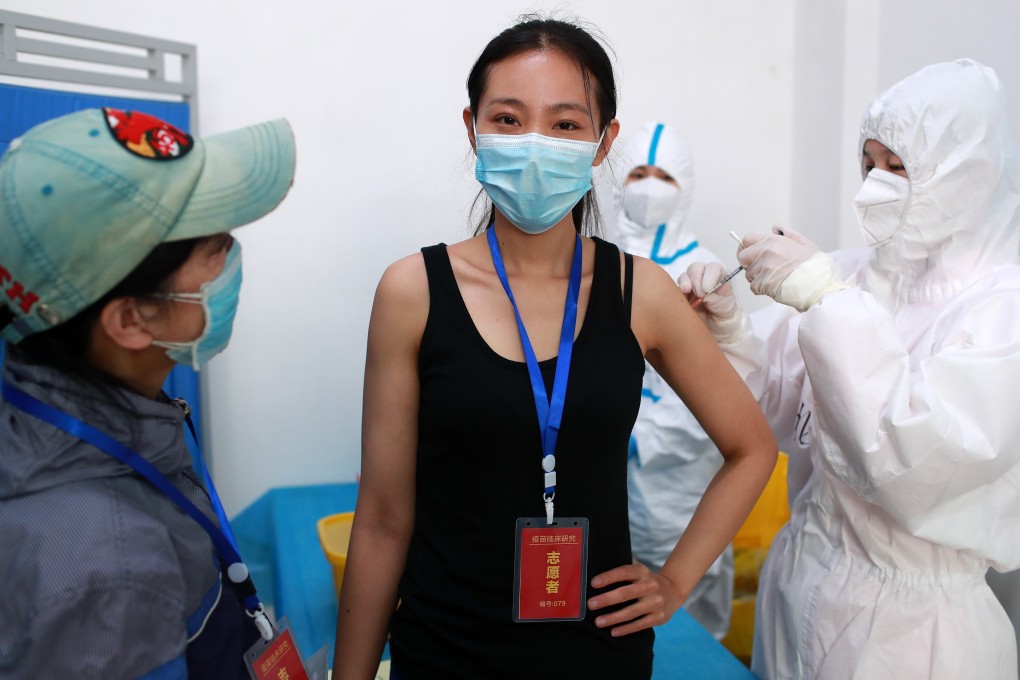
Covid-19 vaccines must have an efficacy rate of 50 per cent and provide at least six months’ immunity if they are to be approved for use in China, the country’s drug regulator has announced.
According to a draft document released by the Chinese Centre for Drug Evaluation (CCDE), 50 per cent is the minimum efficacy rate allowable, although 70 per cent is the target.
The document said also that the regulator would consider granting emergency use of vaccines that have not yet completed their final phase of clinical trials.
Advertisement
Chinese companies are among the forerunners in the race to produce a vaccine for Covid-19, with four candidates in final testing. A total of 29 products are undergoing clinical trials around the world, seven of which are in the final stage.
On Friday, China issued several documents setting out the standards for clinical trials and research on vaccines, including those based on the unproven mRNA platform.
Advertisement
China’s requirement for a minimum 50 per cent efficacy – which means the vaccine would protect half of those injected with it – is in line with the benchmarks set by the World Health Organisation (WHO) and US Food and Drug Administration (FDA).
Advertisement
Select Voice
Choose your listening speed
Get through articles 2x faster
1.25x
250 WPM
Slow
Average
Fast
1.25x