Advertisement
Coronavirus: Peru and Morocco to start phase 3 trials of two Chinese vaccine hopefuls
- Candidates developed by state-owned China National Biotec Group are already being tested in Abu Dhabi, and will also be trialled in Bahrain
- Expert says conducting the trials across multiple locations will speed up the process
Reading Time:2 minutes
Why you can trust SCMP
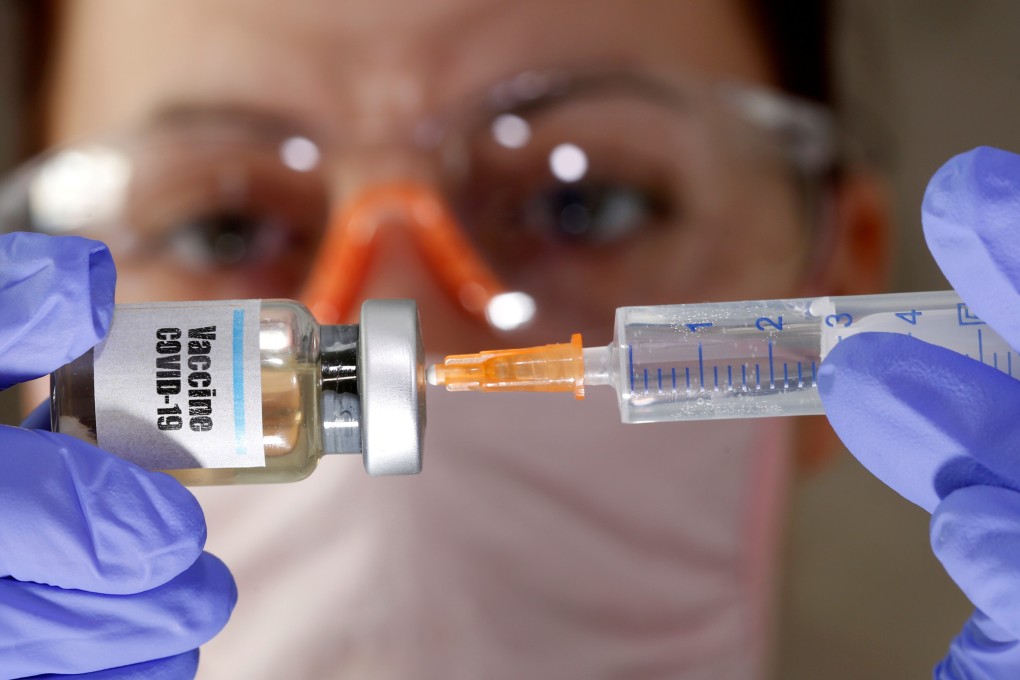
Two Covid-19 vaccine candidates developed by state-owned China National Biotec Group have been approved for phase 3 trials in Peru and Morocco.
Agreements with the two governments were signed in Beijing on Thursday, according to a CNBG statement.
The potential inactivated vaccines were developed by CNBG subsidiaries in Wuhan and Beijing and finished phase 2 trials in June.
Advertisement
Phase 3 tests the efficacy and broader safety of the candidate and the trials usually involve thousands of volunteers.
The experimental vaccines are going through a phase 3 trial in Abu Dhabi at present, with 15,000 volunteers recruited. They will also be tested in Bahrain in a phase 3 trial involving 6,000 people.
06:17
‘Robust immune responses’ found in Covid-19 vaccine clinical trials point to 2021 release
‘Robust immune responses’ found in Covid-19 vaccine clinical trials point to 2021 release
In Peru, the trial will start on Monday with 6,000 volunteers, according to Peruvian state news agency Andina.
Advertisement
Select Voice
Select Speed
1.00x