Coronavirus vaccine developed in China induces quick immune response
- Sinovac’s experimental vaccine findings show CoronaVac suitable for emergency use during pandemic, researchers say
- Unlike other vaccines in development this one could be stored at normal refrigerator temperatures
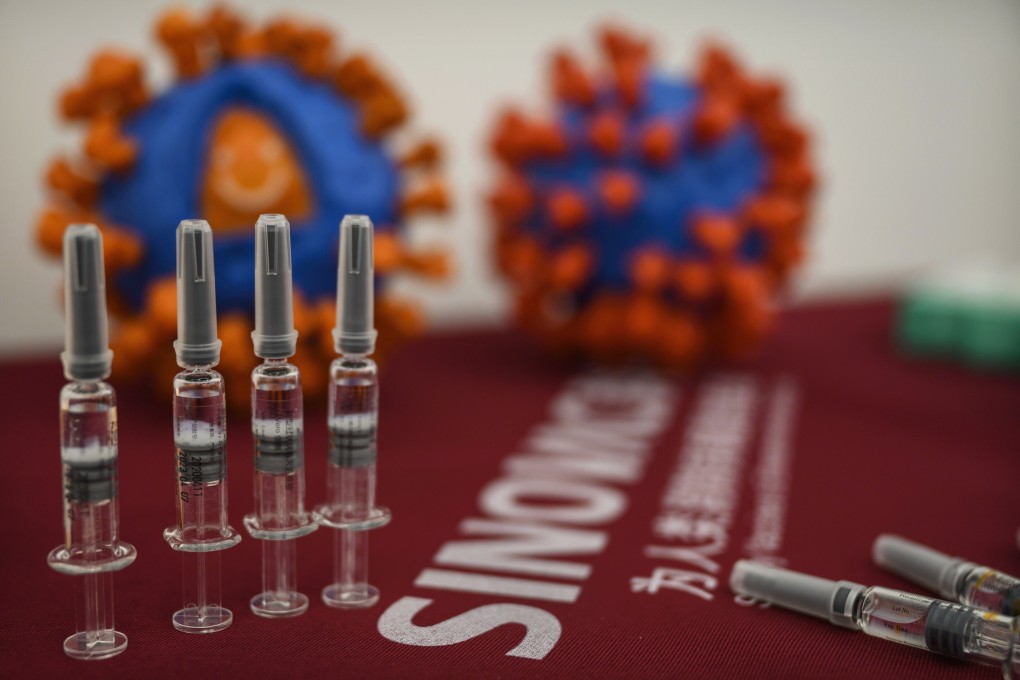
While the early to mid-stage trials were not designed to assess the efficacy of CoronaVac, researchers said it could provide sufficient protection, based on their experience with other vaccines and data from preclinical studies with macaques.
CoronaVac and four other experimental vaccines developed in China are currently undergoing late-stage trials to determine their effectiveness in preventing Covid-19.
The Sinovac findings, published in a peer-reviewed paper by medical journal The Lancet Infectious Diseases, came from results in phase 1 and phase 2 clinical trials in China involving more than 700 participants.
“Our findings show that CoronaVac is capable of inducing a quick antibody response within four weeks of immunisation by giving two doses of the vaccine at a 14-day interval,” Zhu Fengcai, one of the paper’s authors, said. “We believe this makes the vaccine suitable for emergency use during the pandemic,” Zhu said in a statement published alongside the paper.