Chinese vaccine maker hoping to inoculate children as young as three
- China National Biotec Group conducted early-phase trials on children, and reported ‘excellent’ safety data but is continuing to analyse immune response
- Any possible need for booster doses for its vaccine, already approved for adults, will be assessed when data shows how long immunity lasts
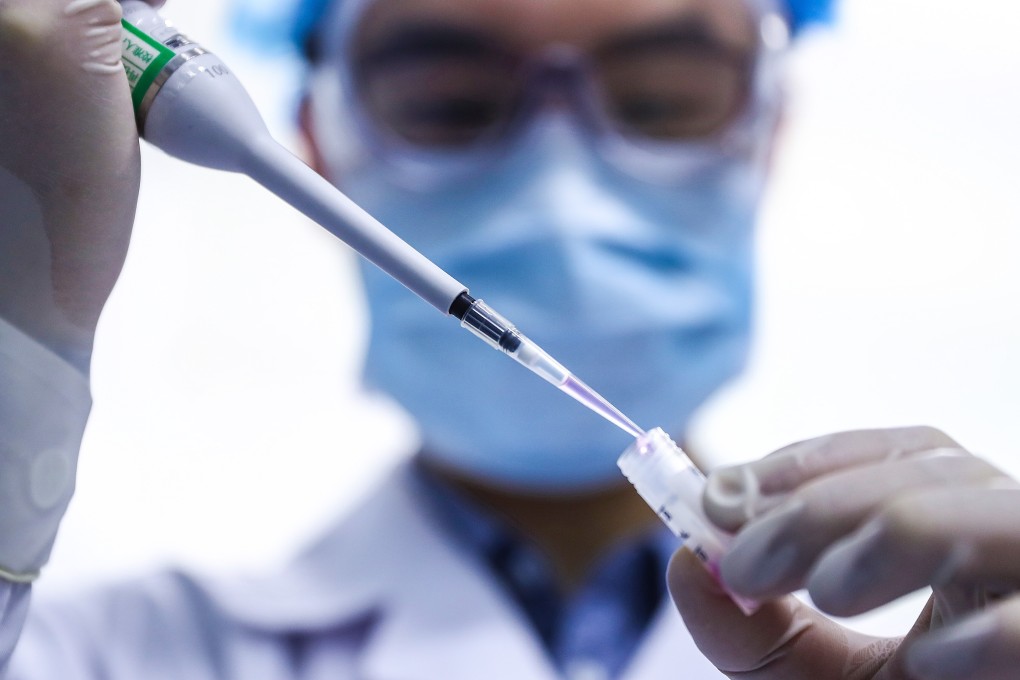
The vaccine was last month approved to be used on 18-to-60-year-olds after interim analysis found it offered 79 per cent protection in its phase 3 trial, conducted on the same age group. It has also been approved for emergency or general use in several other countries, including Egypt, Jordan and the United Arab Emirates.
Vaccines by Moderna and AstraZeneca-Oxford have been approved for emergency use on those aged 18 and over in the United States, European Union and Britain, while the one made by Pfizer-BioNTech is approved to be used on people 16 years old and above.
09:50
SCMP Explains: What's the difference between the major Covid-19 vaccines?
Zhang said CNBG, a subsidiary of Sinopharm, had completed phase 1 and 2 trials on three younger age groups – ages 12 to 17, five to 12, and three to five – to establish whether the vaccine was safe and could trigger an immune response.
“The safety data is excellent but the data for immunogenicity is still being tested,” he said.