China’s production bottleneck ‘could be eased with latest Covid-19 vaccine’
- Emergency use approval paves way for large-scale manufacture of cheap, easy-to-produce vaccines but three doses required
- Non-peer reviewed clinical trial data suggests vaccine is well tolerated and produces immune response against the virus

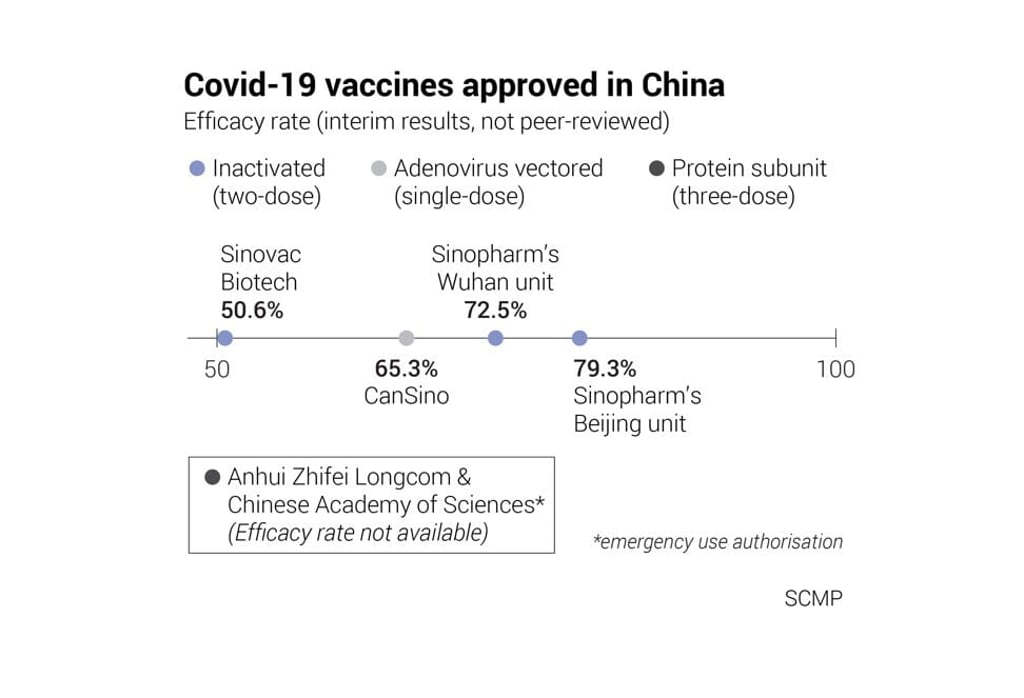
No interim results for the final stage clinical trials – involving 29,000 people – have been released but non-peer reviewed data from phase 1 and 2 trials found the vaccine, jointly developed by Anhui Zhifei Longcom Biopharmaceutical and the Chinese Academy of Sciences’ Institute of Microbiology, to be “well-tolerated and immunogenic”.
The same data, published in December on the preprint server medRxiv.org, showed no serious adverse events related to the vaccine, and indicated that neutralising antibodies were detected in 97 per cent of participants in a group receiving 25 microgram doses in phase 2 trials.
China’s regulatory authority, the National Medical Products Administration, granted similar emergency use approvals (EUAs) based on phase 1 and 2 trial data to three inactivated vaccines in July last year. A fourth vaccine was approved by the military’s drug regulatory authority in China.