China may approve first Covid-19 drug within weeks
- Monoclonal antibody treatment developed by Chinese and US researchers might get conditional approval by year-end, science and technology ministry daily says
- Sinopharm subsidiary meanwhile is readying clinical trials for both human and lab-made antibody treatments
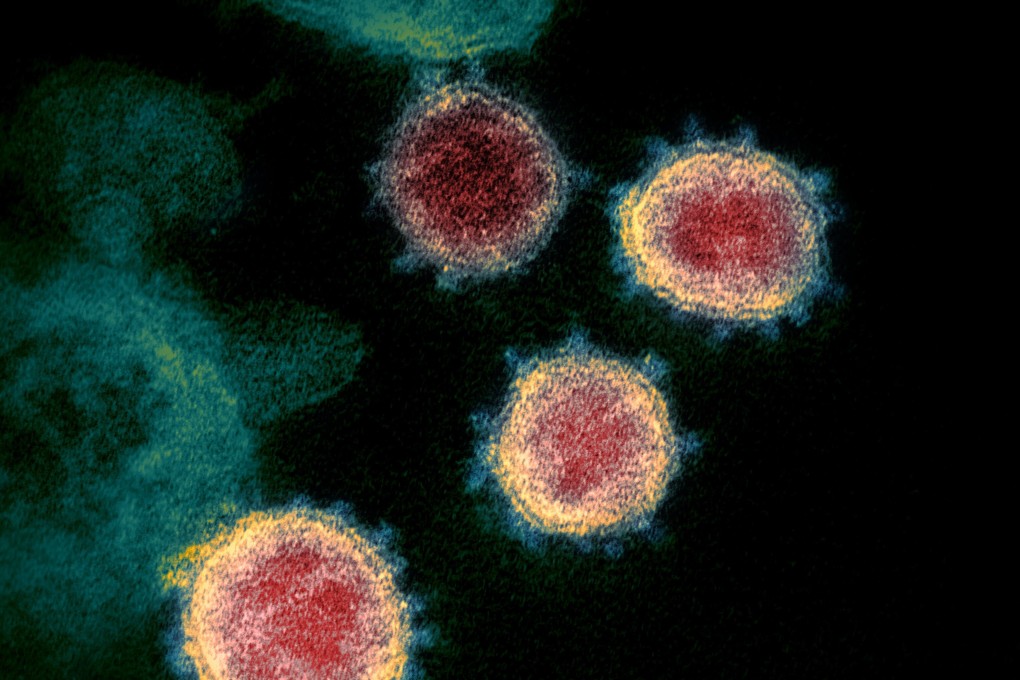
Conditional approval may be given to a neutralising monoclonal antibody treatment co-developed by Chinese and US researchers by the end of the year, according to an official publication of the Ministry of Science and Technology.

01:10
Joy as US borders reopen to fully vaccinated visitors after 20 months of Covid-19 restrictions
Brii Biosciences, a multinational pharmaceutical firm based in the US and China, developed the therapy with scientists at Tsinghua University and the Third People’s Hospital of Shenzhen, Science and Technology Daily reported on Monday.
Monoclonal antibodies are laboratory-made protein molecules that can act like human antibodies. The Brii Bio treatment combines two neutralising monoclonal antibodies, BRII-196 and BRII-198, created by cloning and combining antibodies found in recovered patients in China. The combination therapy has to be administered by infusion.
Brii Bio applied to the Chinese drug regulator last month after phase 3 trials delivered promising interim data. The company was also seeking emergency use clearance in the US, a spokeswoman said.
“We hope the Chinese regulator will approve it by the end of this year,” she said.