Nasal spray provides nearly 80 per cent protection against Covid-19, study finds
- But the antibody spray – developed by Sinovac – has side effects, and experts say more details are needed
- It was given to thousands of health workers in northern China for the study, which has yet to be peer-reviewed
Thousands of health workers were recruited for the study in Hohhot, Inner Mongolia during a Covid-19 outbreak in November. Those who used the nasal spray twice a day got infected at about one-fifth the rate of those who did not, a team of researchers reported in a paper posted to preprint server medRxiv on Saturday.
The spray was developed by Sinovac Life Sciences and contains a broad-spectrum antibody known as SA58 to neutralise the coronavirus – including all known Omicron strains, according to the company. It caused about 1,800 adverse events, such as a runny or dry nose and sneezing, during the study.
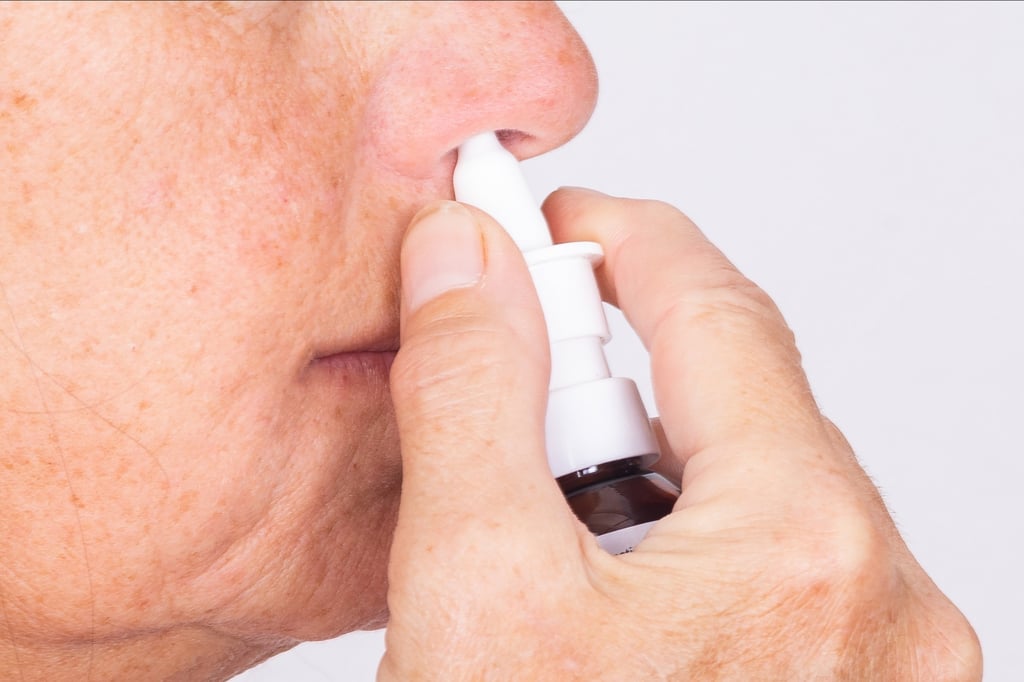
But the adverse events “were all mild and disappeared quickly without affecting daily work”, the authors from Sinovac, Peking University, the Chinese Centre for Disease Control and Prevention, Inner Mongolia Blood Centre and Inner Mongolia Fourth Hospital wrote in the paper.
“This clinical study of the SA58 nasal spray on medical personnel showed good tolerance and good effectiveness for preventing Covid-19 infections, suggesting further application in other populations in the real world,” the team said.
Their paper, which is awaiting peer review for publication, has attracted attention from around the world.
Donna Farber, an immunologist at Columbia University in New York, said the study was “interesting, but not well controlled and there isn’t enough information provided to accurately evaluate the findings”.