China’s new bioethics guideline ‘most comprehensive’ since He Jiankui gene-edited baby scandal
- Latest rules on life sciences and medical research involving humans expand regulatory scope of 2016 guideline and spell out administrative penalties
- Analysts hail move as ‘most important’ since gene-edited baby row but warn of systemic vulnerabilities such as the lack of an open academic culture
The regulation, released by four Chinese ministries earlier this week, comes some seven years after the first such guideline was issued by Beijing.
The latest version widens the regulatory scope of the 2016 guideline, and includes more details on informed consent for human-related research and the administrative penalties for violations.
Specifically, the 2016 guideline only applied to biomedical research in medical institutes, but the new one covers human-related research not only in medical institutes, but colleges and research institutions as well.
Such bodies should set up an ethics review committee if they conduct life sciences and medical research involving humans, according to the regulation.
The new guideline also expands the areas of research that require ethical review.
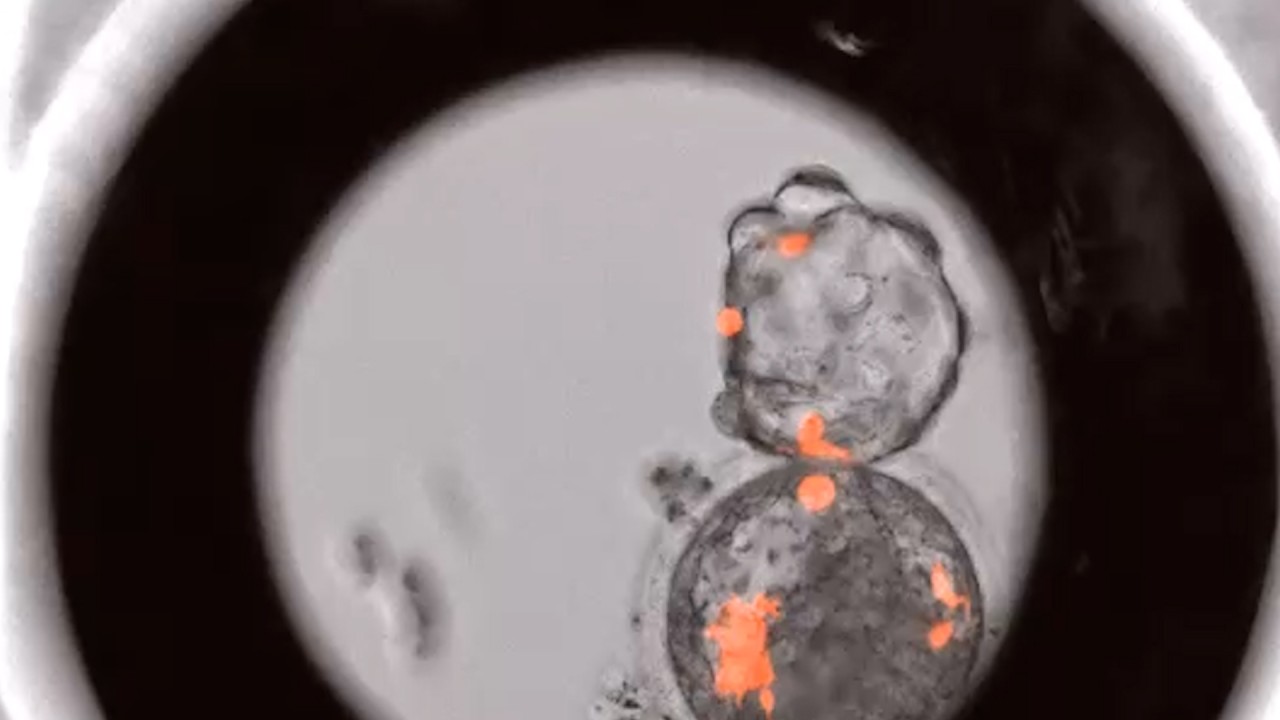
Apart from conventional human subject research, studies into human cells, tissues, organs, fertilised eggs, embryos and fetuses will also be subject to such review.
The document containing the guidelines – titled “Measures of the Ethical Reviews of Life Science and Medical Research Involving Humans” – was released by the National Health Commission, the Ministry of Education, the Ministry of Science and Technology, and the National Administration of Traditional Chinese Medicine on February 28.
Scientists call for ban on human embryo gene edits after He Jiankui scandal
“The promulgation … is arguably the most important and comprehensive national legislation on bioethics China has introduced since 2018,” Joy Zhang, sociologist at the University of Kent, and Lei Ruipeng, bioethicist at the Huazhong University of Science and Technology in Wuhan, wrote in a review published on the Hastings Bioethics Forum website.
But the pair also highlighted the challenges ahead.
“While China has made substantial improvements on its national ethics review regulation, vulnerabilities remain,” they wrote.
In addition to legislative updates, nurturing a culture where Chinese academics “feel comfortable to have open and frank conversations on challenging questions”, both within the country and overseas, was also critical for ethical oversight, they said.
The guideline also requires special protection for research participants from specific vulnerable groups, such as children, pregnant women, the elderly, and those with intellectual or mental disabilities.
“Special attention should be given to [research] involving fertilised eggs, embryos, fetuses or those that may be affected by assisted reproduction techniques,” the rule says.
Institutions and their ethics review committees will also be penalised if they fail to file and upload information on the national medical research registration system, or fail to have researchers submit relevant reports and conduct follow-up reviews.
This comes as advances in biotechnology, artificial intelligence, big data and other emerging technology pose increasing challenges for ethical governance.
“Technology is a powerful tool for development, but it can also be a source of risk,” Chinese President Xi Jinping said in 2021.
China’s ChatGPT-like AI should have age limits, ‘two sessions’ delegate says
“It is important to look ahead and assess … social risk and ethical challenges posed by technological development, and improve relevant laws and regulations, ethical review rules and regulatory frameworks.”
China has strengthened its ethics laws and regulations since the gene-edited baby scandal in 2018, which saw Shenzhen-based researcher He slapped with a three-year jail term for “illegal medical practices”.
A national ethics committee for science and technology was set up in 2019 to promote the systematic improvement of ethical governance.
And in March last year, China issued its first comprehensive guideline on enhancing ethical governance in science and technology, including monitoring and early warning of ethical risks, and serious investigation and punishment of violations.