Could this advance in radionuclide therapy help late-stage cancer patients?
- Improvements in treatment method deliver cancer-killing drugs to tumour cells while avoiding healthy tissue
- Chinese university says the work amounts to a disruptive technology in nuclear medicine design
In a new study, researchers engineered radiopharmaceuticals to allow them to “irreversibly” bind radioactive molecules to target proteins in tumour cells. When used in radionuclide therapy, it means the molecules would kill the cancerous cells, and not the healthy ones.
The university said it was the first original research paper in the field of radionuclide therapy that Nature had published in nearly 50 years.
According to the paper, targeted radionuclide therapy has improved outcomes for cancer patients by delivering “potent radionuclides to tumours for localised irradiation”.
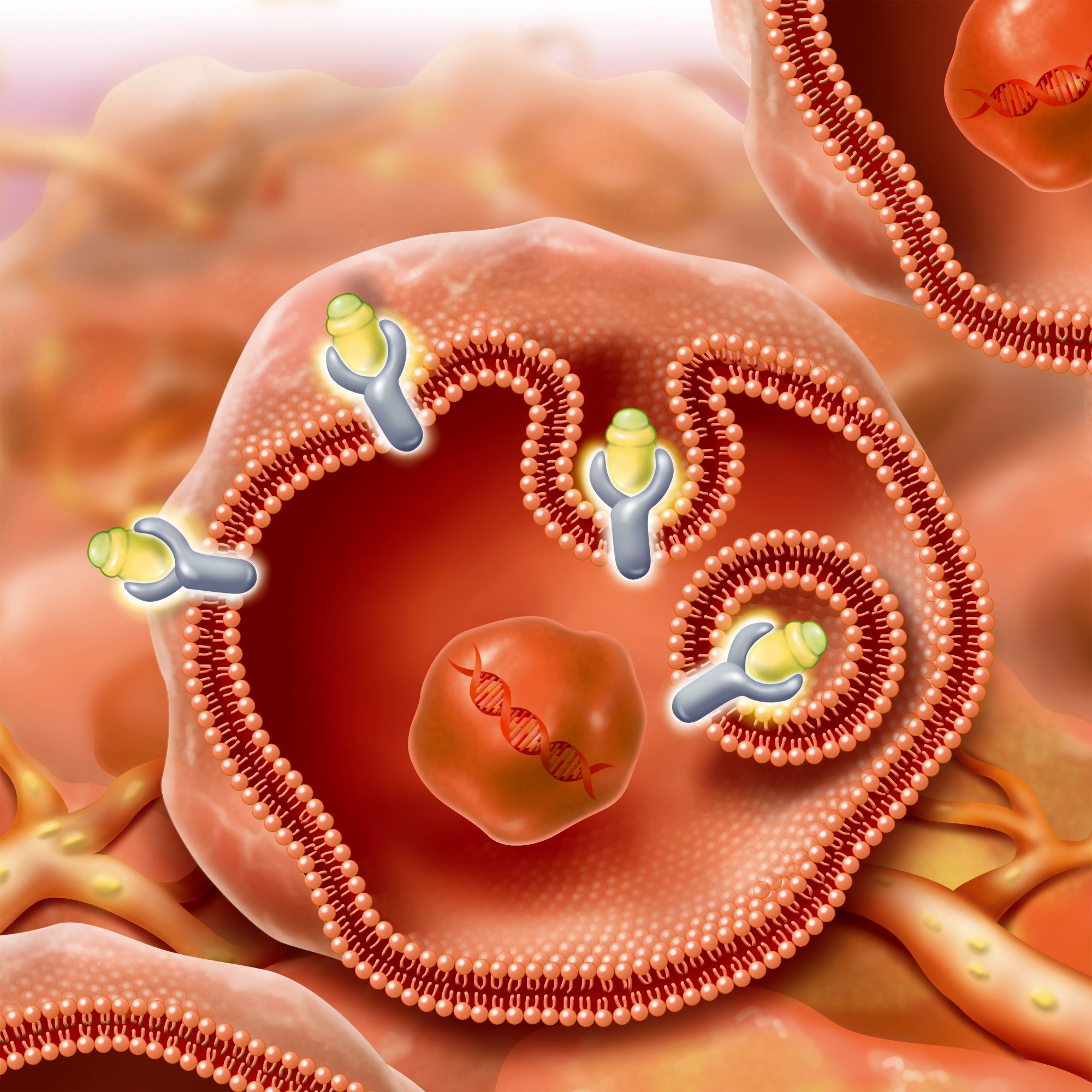
The therapy relies on radioligands, made up of the therapeutic radioisotope and the cell-targeting compound. These radioligands “bind to cancer-associated or specific targets with high affinity”, the team wrote.
However, there are challenges with the technology.
“A therapeutic radiopharmaceutical must achieve both sustainable tumour targeting and fast clearance from healthy tissue, which remains a major challenge,” the researchers said.
“A platform technology that irreversibly fixes radioligands to cancer targets in a tumour-selective manner, if successfully developed, would present an ideal solution to this problem, but has not yet been established.”
To overcome these challenges, the team used covalent targeted radioligands (CTR) which were engineered with a “covalent warhead” that allow the radioligands to form an irreversible bond with the target protein, according to the paper. The team used a sulphur (VI) fluoride exchange (SuFEx) as the warhead.
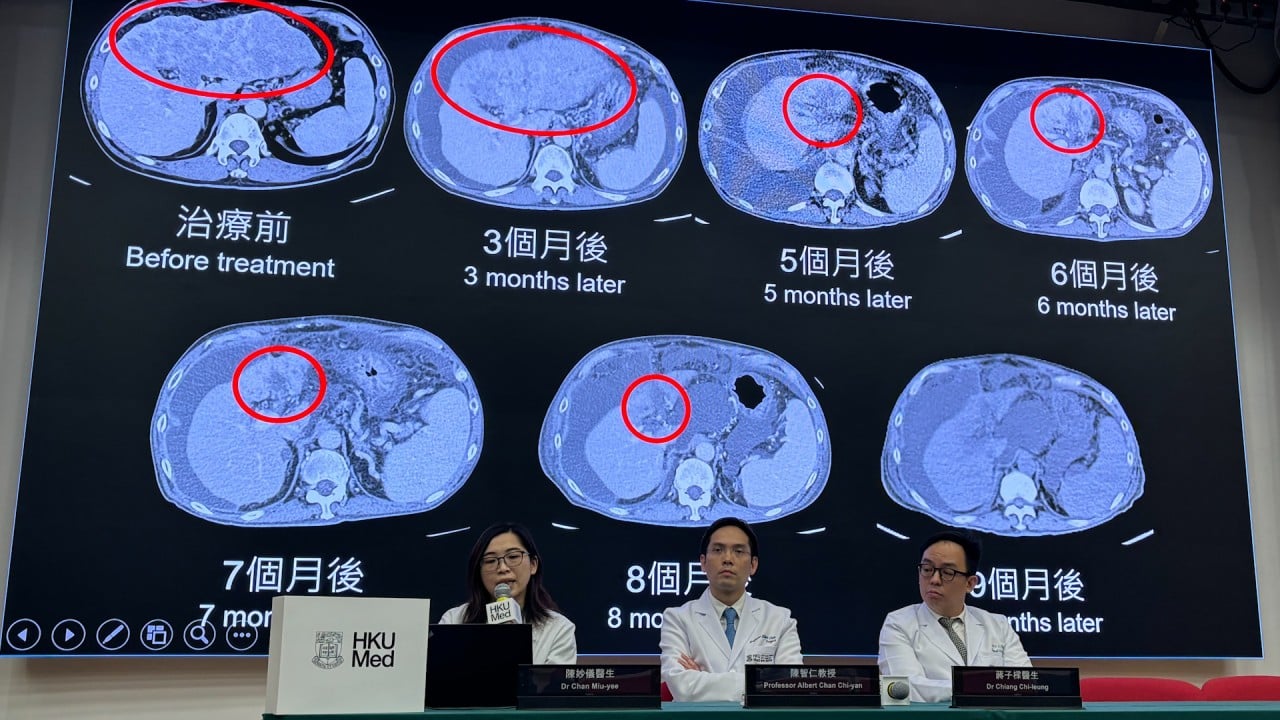
After attaching the warhead to a fibroblast activation protein inhibitor (FAPI), the team found it “triggered more than 80 per cent covalent binding to the [fibroblast activation] protein and almost no dissociation for six days”, the paper said.
The treatment also achieved “nearly complete tumour regression in mice”, the team said.
A major concern surrounding covalent drugs is their potential for binding with sites other than the target.
However, the team found that the SuFEx warhead “might not cause off-target covalent binding in healthy tissues”, based on results of tests in mice, and that any of the drug that does not bind to a target is “rapidly excreted”.
Radioligands can also be combined with a diagnostic nuclide, which can allow a lesion to be visualised using imaging technologies.
The team hope the treatment will be able to be used across a wide range of tumours.
“Considering the broad scope of proteins that can potentially be ligated to SuFEx warheads, it might be possible to adapt this strategy to other cancer targets,” they wrote.