New Chinese Alzheimer’s drug Oligomnnate goes on sale to patients, despite concerns from some medical professionals
- Experimental seaweed-based treatment is now approved for sale but doubts remain about how effective it will be
- It is the first new treatment for disease in 17 years, but regulators have demanded further tests to prove its long-term safety and worth
An experimental Alzheimer’s drug went on sale in China on Sunday despite concerns from many medical professionals.
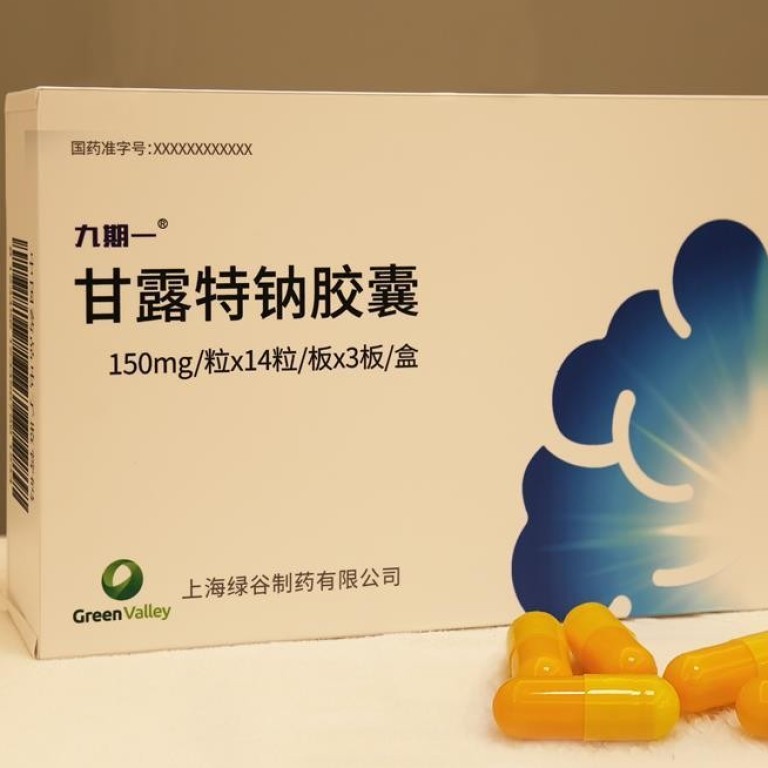
Oligomannate was granted conditional approval by regulators last month and made international headlines as the first new treatment for the disease in 17 years.
However, the drug maker will have to conduct further studies on how the seaweed-based drug works and prove its long-term safety and efficacy.
The Chinese medical community has raised questions about how the drug will actually work, but some patients’ families have said they would clutch at any straws offered.
Using a DNA test to check for disease risk? Doctors alarmed
The drug from Shanghai Green Valley Pharmaceutical Company comes in a 150mg capsule and a month’s treatment will cost 3,580 yuan (US$512), although the makers hope it will eventually be added to the list of subsidised medicines.
According to Alzheimer’s Disease International, China had 9.5 million Alzheimer’s patients in 2015 out of a global total of more than 46 million, and this number is expected to double every 20 years.
On Sunday, Geng Meiyu, a researcher with the Shanghai Institute of Materia Medica who led the discovery and research of the drug, said researchers had submitted data from tests on lab rats to regulators that showed there was no risk of the treatment causing cancer, according to news portal The Paper.

Green Valley also said it planned to recruit more than 2,000 patients with mild-to-moderate Alzheimer’s for 18 months’ of trials in clinical centres in North America, Europe and Asia.
They hope to finish the trials by 2024 and register Oligomannate globally by 2025.
The clinical trials are to be led by Jeffrey Cummings, founding director of the Cleveland Clinic Lou Ruvo Center for Brain Health in the US, who is also a scientific adviser to Green Valley.
“Even if it costs more than 3,000 yuan, we will have to use it, there’s no other way,” the son of one Alzheimer’s patient said.
China approves new Alzheimer’s drug Oligomannate
The 38-year-old named Liu from Hefei in Anhui province said his mother now required constant care and had lost control of her bodily functions and could no longer tell hot from cold.
The family has been using drugs that cost 1,500 yuan a month, but they have seen little improvement in her condition.
The family’s doctor, a woman named Qin, said most Alzheimer’s drugs target beta amyloid, a protein that accumulates in the brain and is strongly believed to disrupt communication between brain cells, eventually killing them.
Green Valley said the treatment works by readjusting levels of bacteria in the gut - a process that is shown to reduce neuron inflammation in the brain and slow the progression of the disease.
The drug uses a sugar unique to seaweed to do so, and its development was inspired by the relatively low occurrence of Alzheimer’s among elderly people who regularly consumed seaweed.
How a 14 year-old Hongkonger built an app for Alzheimer’s patients
However, the mechanism for regulating the bacteria – known as microbiome – is still unproven, Qin said.
She believes that the effectiveness of the drug remains uncertain and has advised patients to wait for more evidence before taking the drug.
However, she said some families were willing to take the change, adding: “They just want to try it and make every possible effort”.

.jpg?itok=H5_PTCSf&v=1700020945)