
Coronavirus: China has been using Covid-19 vaccine candidate on key workers since July, health official says
- Border officials and health workers among first to get jabs as they are more likely to get infected, government adviser Zheng Zhongwei says
- Scheme will later be rolled out to include people working in the transport and service sectors and at wet markets, he says
The government authorised the “emergency use” of the vaccine on July 22, Zheng Zhongwei, director of the National Health Commission’s (NHC’s) science and technology development centre, said in an interview with state broadcaster CCTV.
Zheng, who is also head of an expert panel that advises the government on Covid-19, said the decision to begin inoculating certain groups was “in line with the law”.
He did not say which product had been used or whether the programme involved more than one. Four of the seven vaccine candidates undergoing final phase testing around the world are manufactured by Chinese companies.
Explainer | How do coronavirus vaccines get approved?
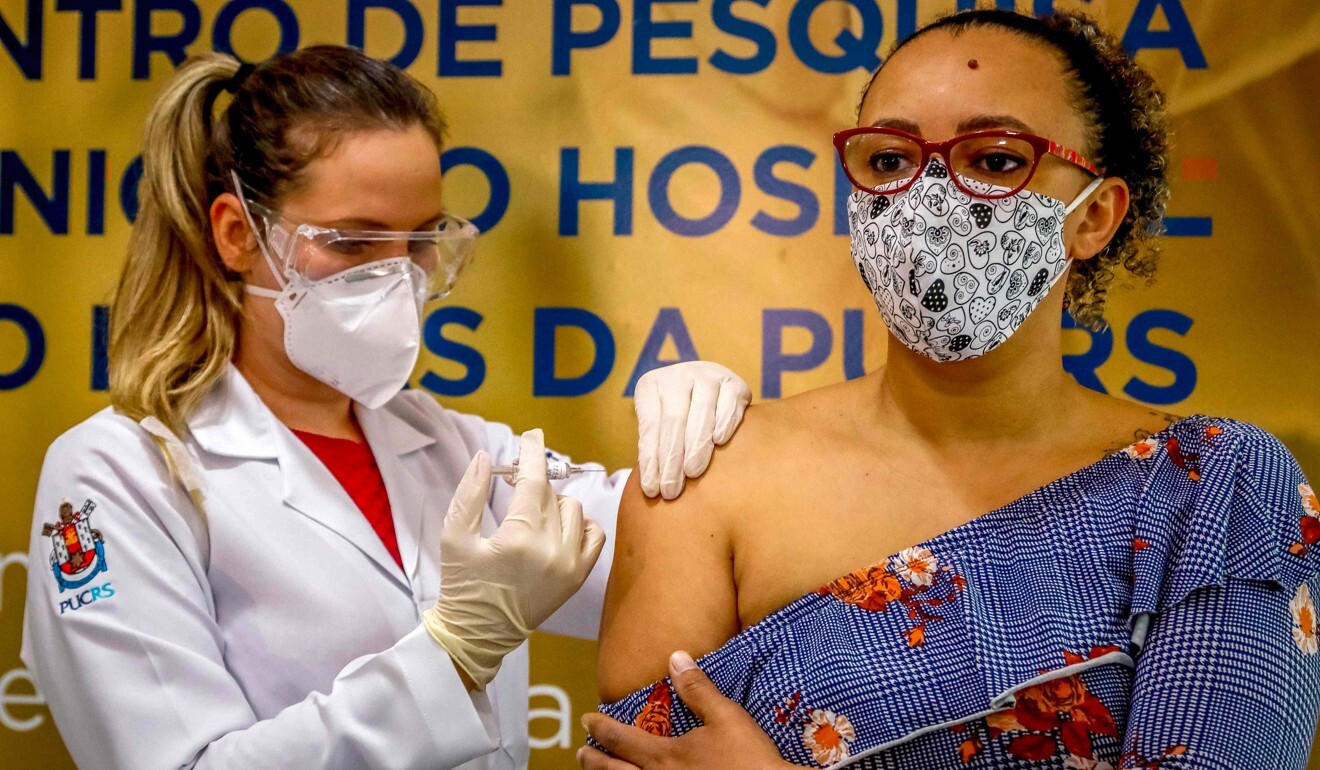
Health workers and border officials were chosen to be among the first people to receive the inoculations as they were more likely than most to get infected with the coronavirus, Zheng said.
“Most cases in China now are imported, so border officials are a high-risk group,” he said.
In the future, the vaccination programme would be rolled out to include people who worked in the transport and service industries and at wet markets, so as to create an “immunity barrier”, he said.
Zheng did not say how many people had been given jabs, but said the next step was to extend the scheme to many more people before the onset of autumn and winter and a possible spike in infections.
The NHC began considering the use of the emergency rule in April but it was not until June 24 that it was given approval to implement it.
A day earlier, China National Biotec Group (CNBG) was given the green light to begin phase three testing of one of its vaccine candidates in the United Arab Emirates. The state-owned company has also been approved to carry out trials in Bahrain, Peru, Morocco and Argentina.
Peru and Morocco to start phase 3 trials of two Chinese vaccine hopefuls
Yang Xiaoming, chairman of CNBG, said 20,000 people had taken part in the overseas trials and the preliminary results were positive.
“The situation regarding the 20,000 volunteers [after being inoculated] shows our vaccines are safe and there have been almost no reports of people suffering side effects,” he said in the same interview as Zheng.
The other Chinese companies involved in final stage clinical trials are Sinovac – in Brazil and Indonesia – and CanSino Biologics in Russia.
The CanSino product was developed in cooperation with the Academy of Military Medical Sciences and in June was approved for use among military personnel.
On the subject of pricing, Zheng said all Chinese Covid-19 vaccines would be “affordable to the public”.
“President Xi [Jinping] said Covid-19 vaccines are public health products. One principle of public health products is that they are priced not based on the supply and demand relationship, but on cost plus a reasonable level of profit,” Zheng said.
“I can tell you that the price will definitely be lower than what chairman Liu said.”
Zhao Dahai, a public health professor at Shanghai Jiao Tong University, said Beijing’s decision to approve the use of vaccine candidates for key workers showed it had confidence in their safety and efficacy.
“It tells the world that we are not playing with the lives of the foreign volunteers taking part in the clinical trials,” he said. “We are quite sure of their safety.”

