Advertisement
Explainer | Could a coronavirus test be as simple and fast as a pregnancy test?
- Without a vaccine, quicker diagnosis and quarantine could be the answer to stopping the pandemic, some scientists argue
- Various technologies are in development but the question is how accurate do they need to be?
5-MIN READ5-MIN
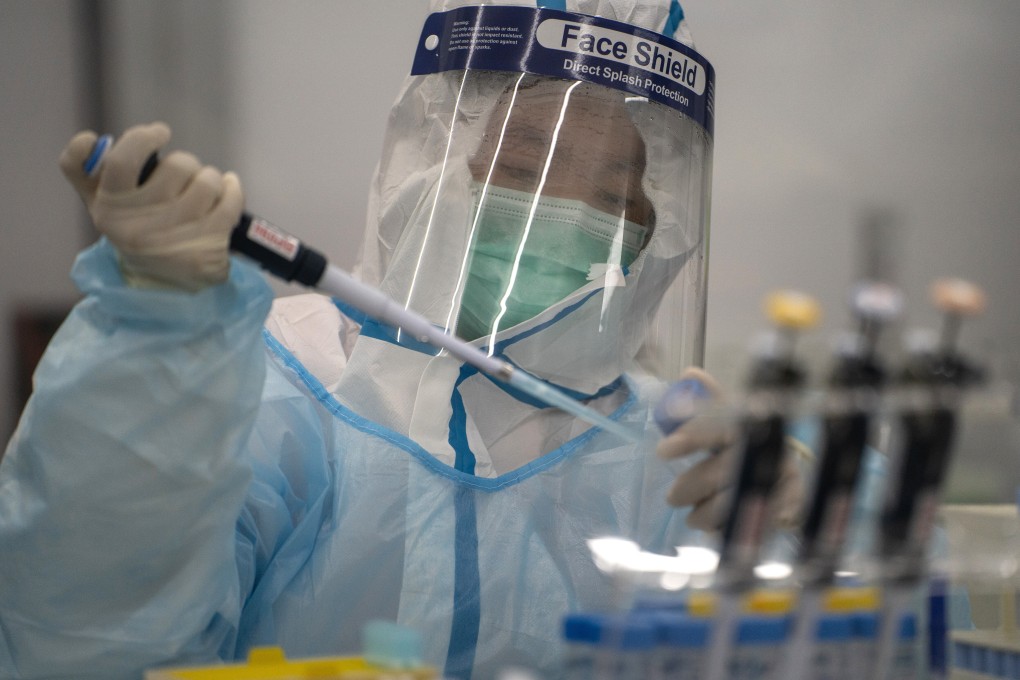
While much of the world’s hopes of overcoming the coronavirus epidemic are on a vaccine, the race is also on to develop a rapid test to detect the pathogen.
Scientists said simple, cheap, at-home kits like pregnancy test sticks could allow people to be tested more often and put in quarantine sooner, making it safer for schools and public activities like football matches to resume, especially before vaccines are available.
Hundreds of different kinds of diagnostic tests are in development and a few have been approved for emergency use.
Advertisement
Conventional RT-PCR tests are still the most accurate but they require expertise and equipment. The debate now is whether some accuracy in testing should be sacrificed for speed and frequency to stop the spread of the coronavirus.
What are PCR tests?
Advertisement
A polymerase chain reaction (PCR) test is a kind of molecular test that detect the genetic material of the coronavirus in a specimen.
The gold standard for diagnosing Covid-19 is the RT-PCR test.
Advertisement
Select Voice
Select Speed
1.00x