
Code of practice and possible new laws on marketing baby milk formula to boost breastfeeding in Hong Kong
Senior health official Sophia Chan reveals voluntary code aims to encourage breastfeeding, as well as expert panel to vet makers’ claims
A voluntary code that restricts the marketing of infant milk formula is set to launch in the next few months, the undersecretary for food and health has revealed.
And a panel of scientists would screen health and nutritional claims by makers of formula before they are allowed to be printed on packaging, if a law regulating the claims is enacted, Sophia Chan added without giving a timetable.
She said such legislation would come in if the code did not improve claims’ veracity.
Chan said the code aimed to encourage breastfeeding and to provide proper product information to mothers.
“We hope to provide effective and proper information to mothers, and to raise the current exclusive breastfeeding rate,” she said.
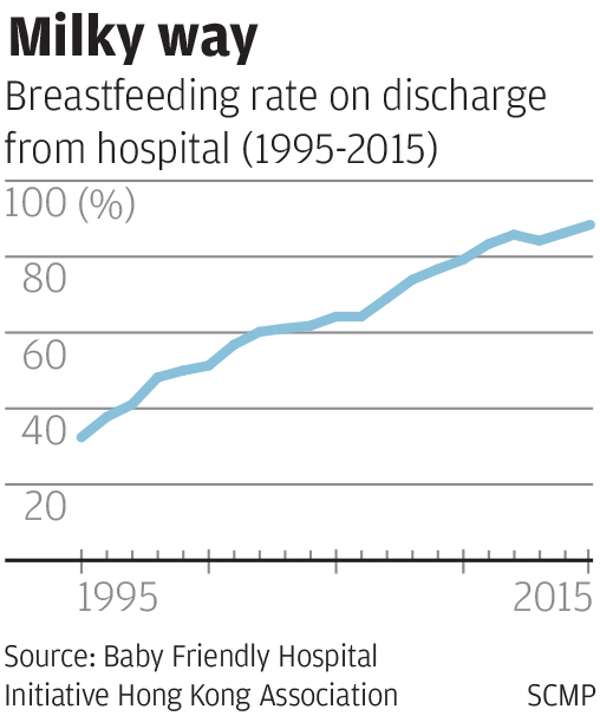
According to the Baby Friendly Hospital Initiative Hong Kong Association under Unicef, some 88 per cent of mothers tried to breastfeed their newborns in 2015.
But separate 2014 figures from the Health Department showed that 31 per cent of mothers managed to feed their babies only with breast milk in the first month of birth.
“Implementing the code voluntarily would be the first step as we want to understand [the market’s response]. We believe traders would not intentionally violate it,” Chan said.
The code, based on international guidelines drafted by the World Health Organisation, would limit formula marketing practices for children under the age of 36 months.
According to the draft, the code would ban promotions for formula, feeding bottles and pacifiers. Advertising and free samples of products would also not be allowed in health care facilities.
The move risks hurting the advertising industry, which saw more than HK$2.5 billion spent on promotions in 2014.
While the code would be voluntary, Chan said legislation would be considered if serious violations were reported.
“We won’t rush to legislation if compliance of the code is good. But we would not rule out legislating the code if many problems surfaced,” Chan said.
A panel of scientists would also be formed to evaluate claims made about nutrition and health under a separate proposal.
“Some claims now are not conclusive or are confusing, but we don’t have a system to assess them,” she said.
But the Hong Kong Infant and Young Child Nutrition Association, which represents seven leading formula makers, said mandatory regulation was preferred to ensure fairness in the market.
They also described the regulation of products for those up to three years old as a “huge step backward”, with some countries, such as New Zealand, doing so only up to the age of six months.
The Post found a variety of claims made in advertising and on the packaging of infant formula.
For example, Abbott claimed that its products contained nucleotides and a prebiotic named GOS that could “enhance immunity” and “support intestinal health”.
Wyeth also said its products for children aged between one and three years old contained a substance named sphingomyelin that could “support brain and cognitive development”.
Dr Ellis Hon Kam-lun, a paediatrics professor at Chinese University, said there would not be much difference in the formula of the various brands despite the claims of companies.
“Some brands may add 10 times more of a particular substance into their milk formula, but there is no scientific evidence showing that babies would get extra benefits,” he said.
He said many claims made by manufacturers regarding the contents of their products concerned things already found in breast milk.
“Indeed there is no need to exaggerate the functions of their products,”Hon said.
Chan said her bureau was seeking advice from the Department of Justice and studying whether the regulatory terms complied with international trade and health standards.
Jannie Leung Hoi-ting, chairwoman of the Breastfeeding Mothers’ Association, supported tighter regulation, saying some mothers or their family members had been misled by the manufacturers of formula.
“Some mothers will receive the wrong message and think milk formula is better than breast milk,” Leung said.
She said in the long run public education would help to raise both acceptance and the knowledge of mothers when it came to breastfeeding.

