Delay in disclosing batch numbers of mouldy Indonesian-made drug Enzyplex ‘intolerable’, Hong Kong pharmacists say
Professional bodies call for tighter checks on imported medication, but health minister says existing system adequate
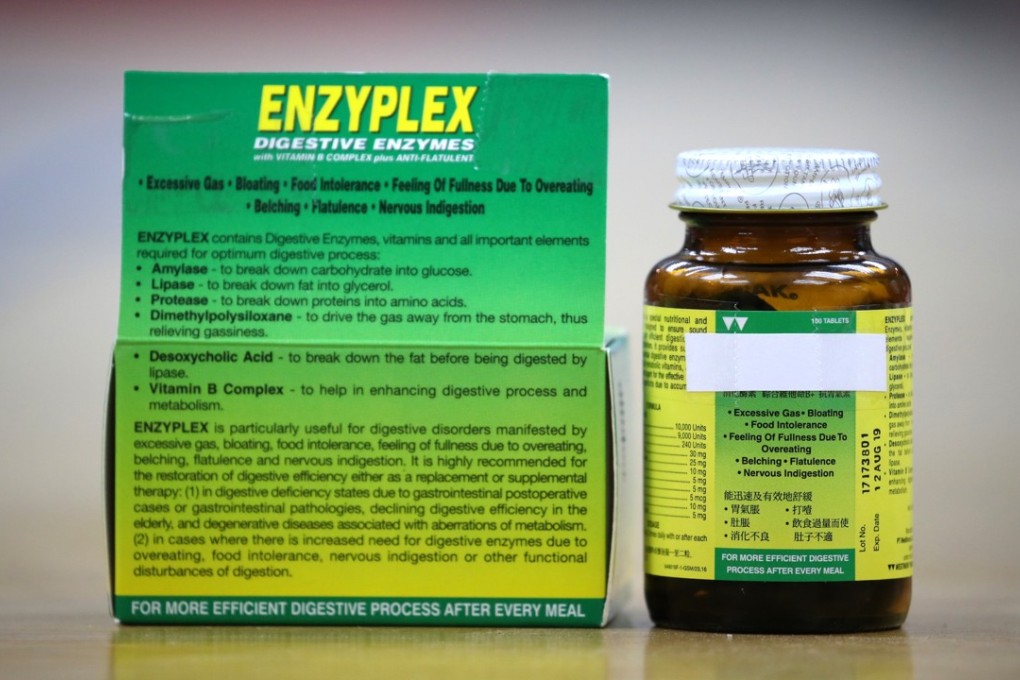
Two professional bodies for Hong Kong pharmacists made public on Saturday the batch numbers of a round of Indonesian-made digestive drugs found to be contaminated with mould, calling the government’s delay in releasing the information “intolerable”.
The Practising Pharmacists Association and the Hong Kong Academy of Pharmacy urged officials to introduce testing at customs checkpoints to catch substandard drugs.
But Hong Kong’s health minister on Saturday insisted the existing system was adequate to protect public health.
The Hospital Authority, the statutory body which manages government hospitals, on Thursday announced that a type of Monascus mould had been found on Enzyplex tablets imported from the Southeast Asian country.

A patient in her 30s who had been suffering from lymphoma died after taking the drug. But the authority said the mould on the tablets was an “incidental” finding when the microbiology department at Queen Mary Hospital in Pok Fu Lam reviewed the deceased woman’s case.
The government’s Department of Health is now investigating the drug.
Iris Chang, the pharmacists association’s president, said the batch numbers in question were 17234801 and 17235101.
She said she had attempted to contact the Department of Health, which oversees drug registrations and import controls, to obtain the numbers but was redirected to the Hospital Authority, which then took two days – over which time more than 10 emails went back and forth – before disclosing the information.