Hong Kong health department issues recall for five heart drugs containing valsartan that was made in China
An estimated 30,000 people could be affected by recall of pharmaceuticals used to treat hypertension and heart failure
Hong Kong’s Department of Health on Friday recalled five prescription drugs that are used to treat heart disease because they have the potential to cause cancer.
An estimated 30,000 people could be affected by the recall of the valsartan-containing drugs, which are prescribed to treat hypertension and heart failure.
The health department instructed wholesalers Actavis Hong Kong Limited and Hong Kong Medical Supplies Limited to recall the medicine from the market after an impurity – N-nitrosodimethylamine (NDMA) – was found in the valsartan, which was produced by a company in mainland China.
NDMA is classified as a probable carcinogen in humans.
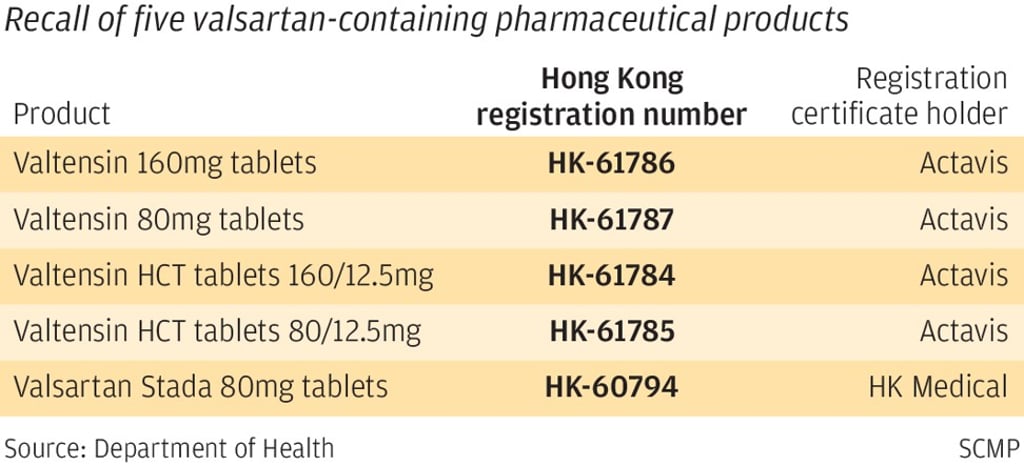
The affected products are Valtensin 160mg tablets, Valtensin 80mg tablets, Valtensin HCT tablets 160/12.5mg, Valtensin HCT tablets 80/12.5mg, and Valsartan Stada 80mg tablets. Four drugs were registered by Actavis with one registered by Hong Kong Medical Supplies.