Hongkongers taking popular blood pressure medication told to carry on, despite fears that it could give them cancer
Drugs provided by city wholesalers found to include NDMA, but doctors warn that patients who stop taking the medicine are putting themselves in danger of having a heart attack or stroke
Medical experts said on Saturday that patients should not stop taking the recalled drug without consulting doctors, as the risk, assessed by the European Medicines Agency in its latest announcement, was not immediate and less than that involved if the patients stopped taking the medication.
The drug was recalled last month after an impurity known as NDMA (N-nitrosodimethylamine) was found in the generic medication valsartan produced by mainland Chinese manufacturer Zhejiang Huahai Pharmaceuticals. NDMA is classified as a probable carcinogen in humans based on laboratory tests.

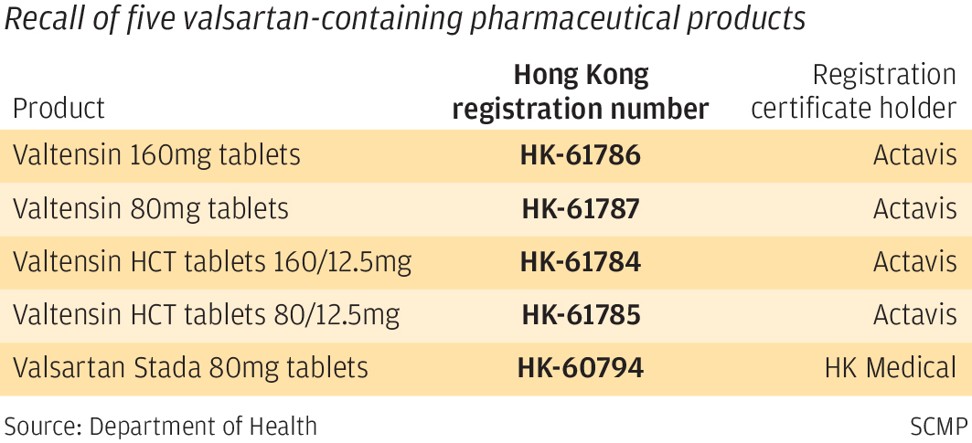
In Hong Kong, five drugs provided by wholesalers Actavis Hong Kong Limited and Hong Kong Medical Supplies Limited were recalled.
The Hospital Authority said about 30,000 patients in public hospitals were prescribed the drug, while local pharmacists estimated that the drugs from the two wholesalers also took up around 30 per cent of the private market share.
On Thursday the agency said that, following a preliminary evaluation, it estimated that “there could be one extra case of cancer for every 5,000 patients taking the affected medicines at the highest valsartan dose every day for seven years”. The agency referred to the highest daily dose as 320mg.
An average 60 ppm of impurity was detected in the raw material valsartan from the mainland Chinese manufacturer. The cancer risk estimation by the European agency was based on this figure, and animal studies.
The estimate was also based on the assumption that NDMA present in the raw material was carried over in the final drug product in the same amount.
William Chui Chun-ming, president of the Society of Hospital Pharmacists of Hong Kong, said that, based on the latest estimates, the cancer risk was low.
“The maximum dose taken by Hong Kong patients seldom reaches 320mg,” he said. “Hong Kong’s patients usually take up to 160mg of the drug per day.”
However, he said it was possible to see a patient taking the drug daily for seven years or more.
“Despite the low risk, it is still possible that some people would be affected,” Chui said. “Further analysis is needed to see how much [harmful substance] each pill contains.”
It is believed NDMA was an “unexpected impurity” formed as a side product after Zhejiang Huahai introduced changes in its manufacturing process in 2012.
But health authorities in Hong Kong and overseas said patients should consult their doctor before they stopped taking the drug.
Dr Li Shu-kin, immediate past president of the Hong Kong College of Cardiology, said the risk from stopping the medication without professional advice could be greater than continuing to take the medicine.