Hong Kong health authorities issue recall of high blood pressure drug over fears batch has been infected with impurity that could cause cancer
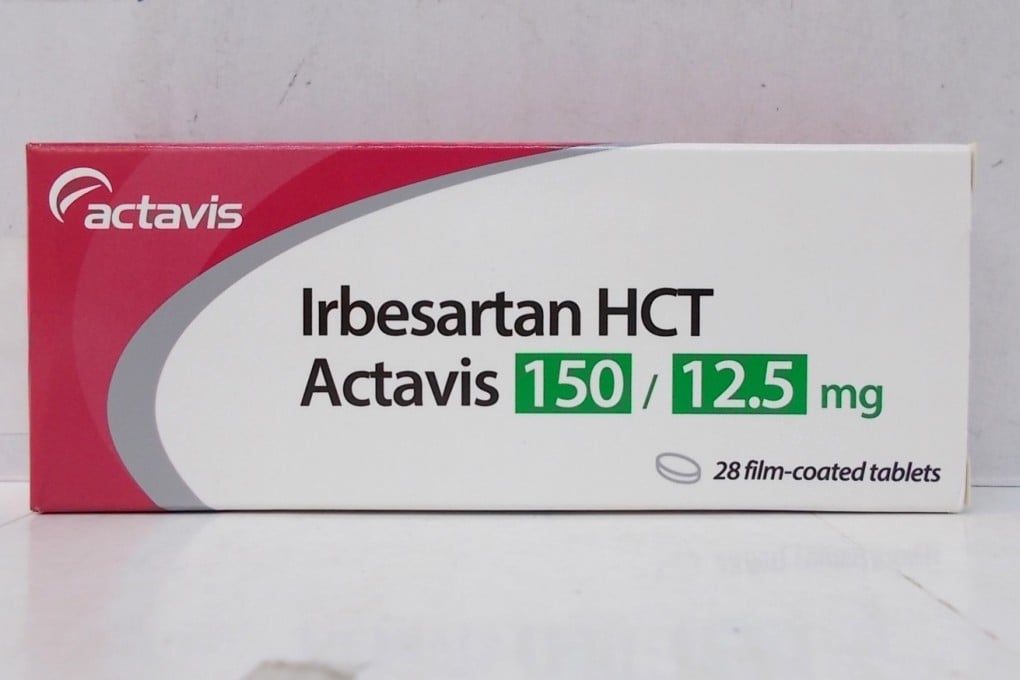
- Irbesartan HCT tablets, with batch number 058818, which were supplied locally by Actavis Hong Kong are subject to recall
- Certain batches of the drug’s raw materials were found to contain N-nitrosodiethylamine (NDEA), a probable human carcinogen
Health authorities in Hong Kong have recalled a batch of a prescription drug used to treat high blood pressure after it was found to contain an impurity that could cause cancer.
The affected product, Irbesartan HCT Actavis Tablets 150/12.5mg, with Hong Kong registration number HK-63378, has been supplied to local private doctors, pharmacies and a private hospital, according to the medicine wholesaler Actavis Hong Kong.
About 3,600 packs, containing 28 tablets each, were said to be on the market. The drug has the batch number 058818.
Thursday’s recall followed a report by Actavis to the Department of Health that certain batches of the drug’s raw materials were found to contain N-nitrosodiethylamine (NDEA).
NDEA is classed as a probable human carcinogen, a substance that could cause cancer.
In a statement, the department said: “A number of irbesartan-containing products using these raw materials were thus affected. Among the affected products, the [batch of tablets in question] has been imported into, and supplied in, Hong Kong.