Advertisement
Coronavirus Hong Kong: US drug maker Moderna says bivalent Covid vaccine approved for local use, marking second such jab available in city
- German-based BioNTech’s jab, targeting Omicron subvariants BA.4 and BA.5, introduced last December
- Sinovac and CanSino Biologics to provide first-generation vaccines to private market this month to cater for those ineligible for free booster shots
Reading Time:3 minutes
Why you can trust SCMP
1

US pharmaceutical giant Moderna on Wednesday said its bivalent Covid-19 vaccine had been approved by the Hong Kong government for local use, marking the second such jab available in the city.
The approval came as two Chinese drug makers Sinovac and CanSino Biologics introduced their first-generation vaccines to the city’s private market.
Moderna said the Department of Health had granted marketing authorisation for the use of its vaccine targeting Omicron subvariants BA.4 and BA.5.
“This decision provides another Covid-19 vaccine option in Hong Kong and highlights our commitment to bring innovative mRNA vaccines and therapeutics to address unmet healthcare needs in the market, based on our leading mRNA platform,” said Sian Ng, the firm’s Hong Kong general manager.
Advertisement
“We are grateful for this approval decision and are committed to bringing this vaccine to people in Hong Kong as soon as possible.”
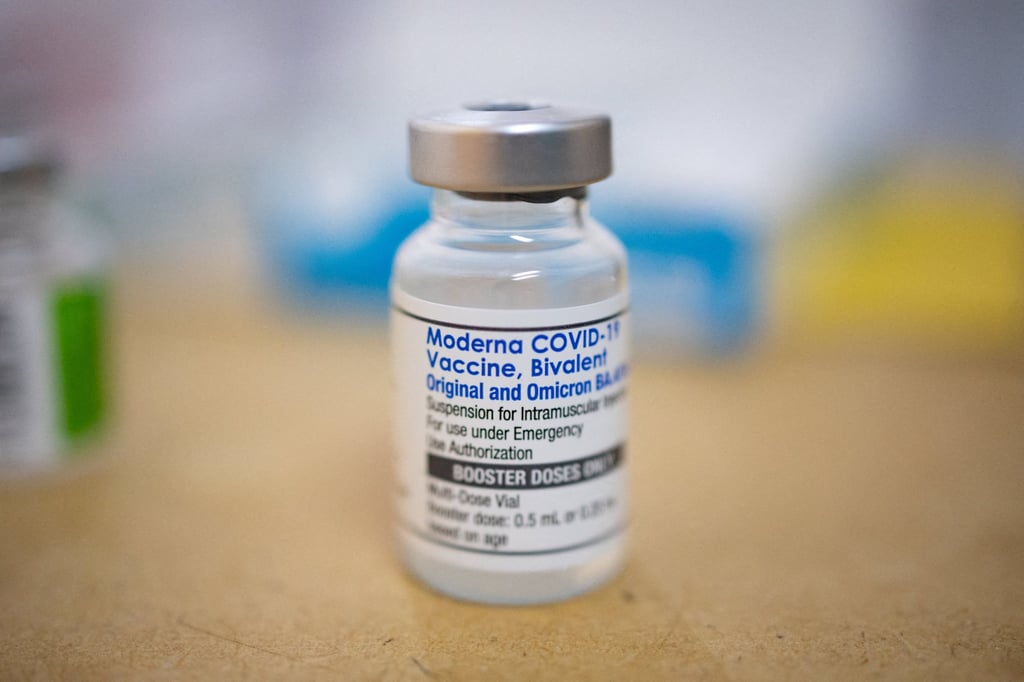
The German-made BioNTech bivalent jab, targeting Omicron, was the first such vaccine to be approved for use on residents aged 12 or older in Hong Kong last December.
Advertisement
Advertisement
Select Voice
Choose your listening speed
Get through articles 2x faster
1.25x
250 WPM
Slow
Average
Fast
1.25x