Advertisement
Coronavirus: Russia starts making second Covid-19 vaccine as cases rise
- Output of the second vaccine, which has yet to complete trials, will ramp up by the end of the year, says the head of Russia’s public health watchdog
- Unlike Sputnik V, Russia’s second vaccine is a mix of short amino-acid chains, called peptides, that induce an immune response
Reading Time:3 minutes
Why you can trust SCMP
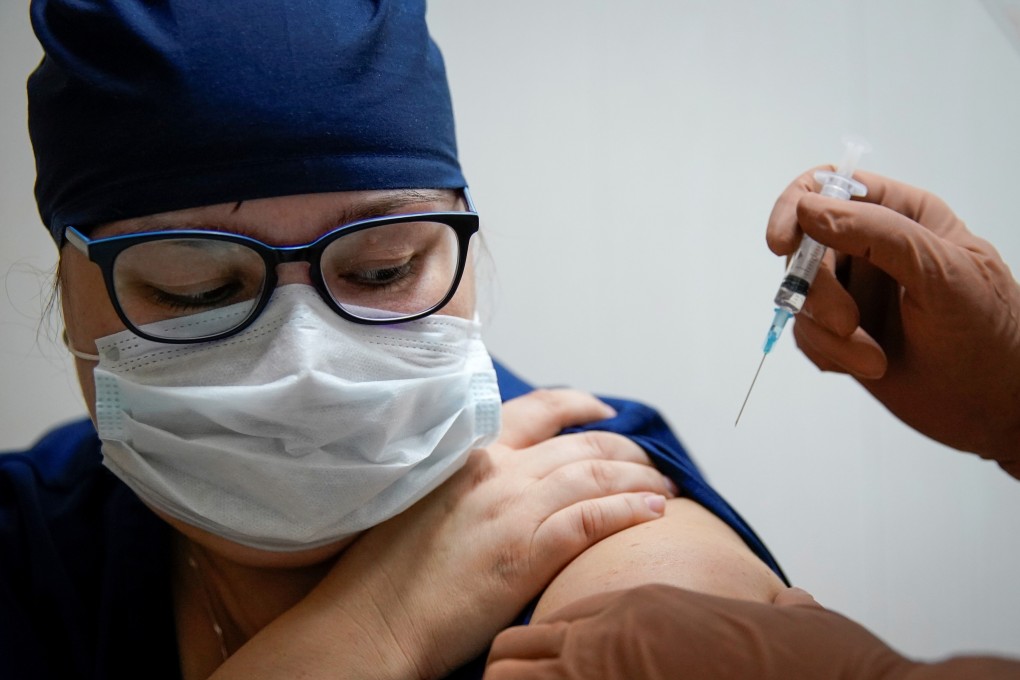
Russia has begun production of a second coronavirus vaccine that has not completed trials, as the Kremlin rushes to develop a shield against the pandemic.
Output of the Covid-19 vaccine, developed by former biological weapons lab Vector State Virology and Biotechnology Centre in Novosibirsk, will ramp up by the end of the year, said Anna Popova, the head of Russia’s public-health watchdog, at a conference on Tuesday.
President Vladimir Putin announced the approval of Vector’s vaccine earlier this month, following a similar trajectory of the Sputnik V inoculation in August, which he claimed was the first to be registered in the world.
Advertisement
Both were tested on a limited number of people before receiving provisional registration that will allow for widespread use as they undergo Phase 3 trials to prove they are safe and effective.
Advertisement
The authorities are hoping the experimental vaccines will stem the tide of the pandemic while minimising the economic fallout that a lockdown would entail.
Advertisement
Select Voice
Choose your listening speed
Get through articles 2x faster
1.25x
250 WPM
Slow
Average
Fast
1.25x
