Coronavirus: Turkey approves China’s Sinovac vaccine for emergency use
- The country’s health minister and members of the country’s scientific advisory council received the first shots live on television
- Widely differing efficacy rates have been reported for the CoronaVac shot from clinical trials in Turkey, Brazil and Indonesia
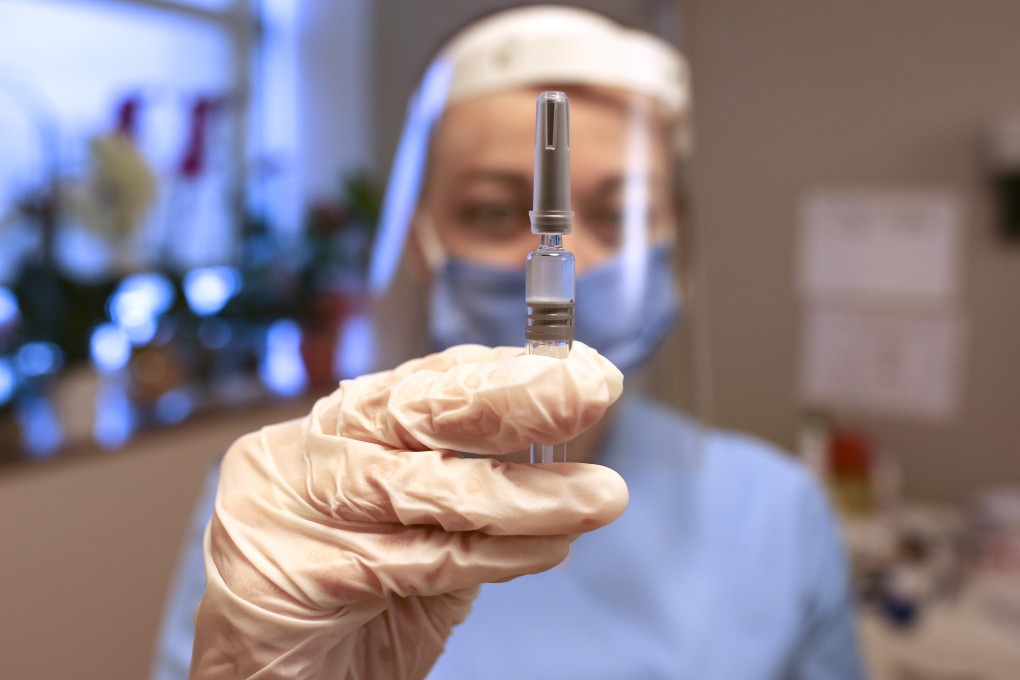
Turkish authorities gave the go-ahead for the emergency use of the Covid-19 vaccine produced by China’s Sinovac Biotech on Wednesday, paving the way for the roll-out for Turkey’s vaccination programme starting with health care workers and other high-risk groups.
The country’s health minister and members of the country’s scientific advisory council received the first shots live on television, soon after the health regulatory authority, the Turkish Medicines and Medical Devices Agency, announced it had given its approval for CoronaVac’s emergency use in the country of 83 million.
02:27
Erdogan gets jab as Turkey rolls out coronavirus vaccine campaign with China’s Sinovac
“I had previously said that there is light at the end of the tunnel,” Koca said as he received the first dose of the vaccine, which will be delivered in two doses. “I believe that the days ahead of us will be bright.”
Koca said Turkey’s vaccination programme would begin on Thursday, starting with health care workers. He urged all citizens to be vaccinated, saying it was the most promising way to beat the pandemic.
The shots would carry a QR code assigned to a person’s name in accordance with Ankara’s vaccination programme and an online appointment system.
The Sinovac vaccine underwent clinical trials in Turkey, Brazil and Indonesia, which have all reported diverging efficacy rates.
In December, Turkish authorities announced an efficacy rate of 91.25 per cent from interim analysis of 29 cases in a trial with 7,371 volunteers. About 12,450 volunteers, including 1,500 health care workers, are involved in the late-stage clinical trials.