Coronavirus: China and India may produce Russia’s Sputnik vaccine, Vladimir Putin says at BRICS summit
- BRICS leaders, including Modi and Xi, vow that when Covid-19 shot becomes available, it will be ‘disseminated in a fair, equitable and affordable basis’
- Brazil to receive first doses of China’s Sinovac vaccine this week
Russian President Vladimir Putin told BRICS leaders on Tuesday that coronavirus vaccines developed in Russia “work effectively and safely” and urged the group of emerging economy nations to “join forces” for the mass production of the shots.
Putin’s remarks come after early results of large studies of several experimental Covid-19 vaccines, including a Russian one, were announced. The BRICS grouping is made up of Brazil, Russia, India, China and South Africa.
The Russian Direct Investment Fund, which bankrolled the development of Sputnik V, has agreements with India and Brazil to conduct trials of the jab, and with pharmaceutical companies in India and China to produce it, the Russian leader said.
“I think this is very important,” he added.
02:23
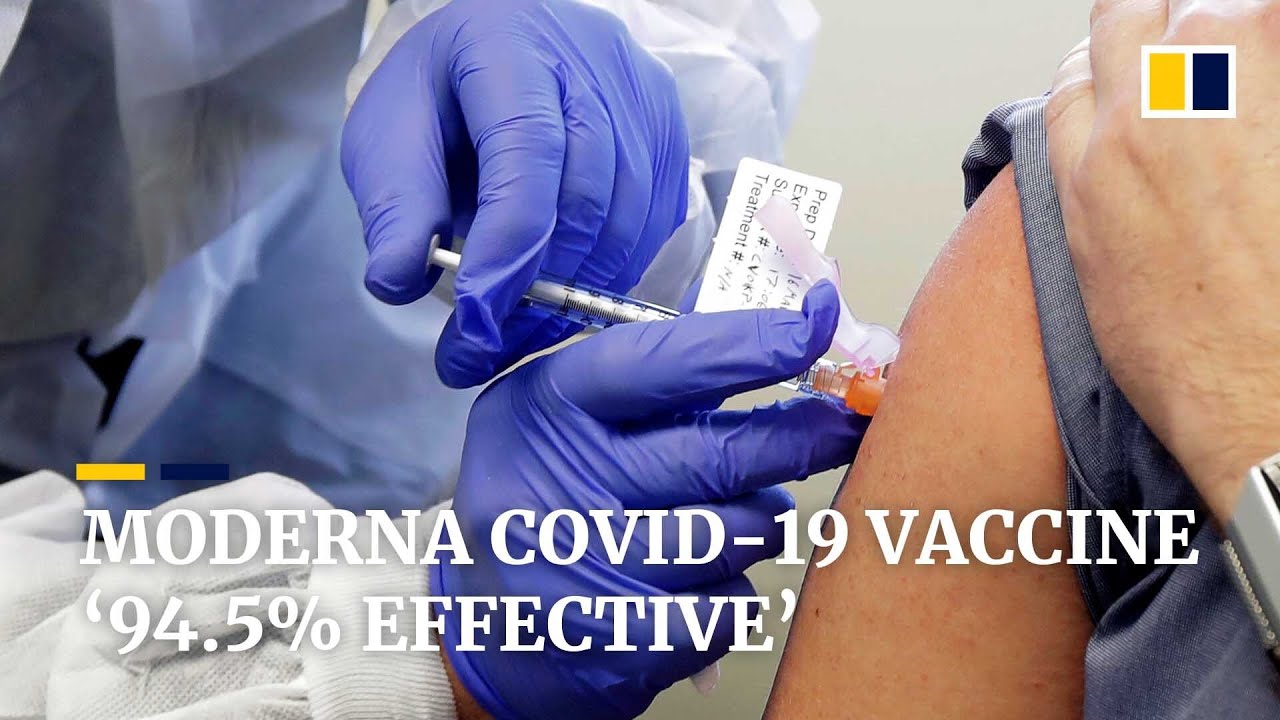
Moderna Covid-19 vaccine nearly 95 per cent effective in second promising trial for US drug makers
Moderna said on Monday its vaccine appears to be 94.5 per cent effective, according to preliminary data from an ongoing study. Last week, Pfizer said its own vaccine looked 90 per cent effective.