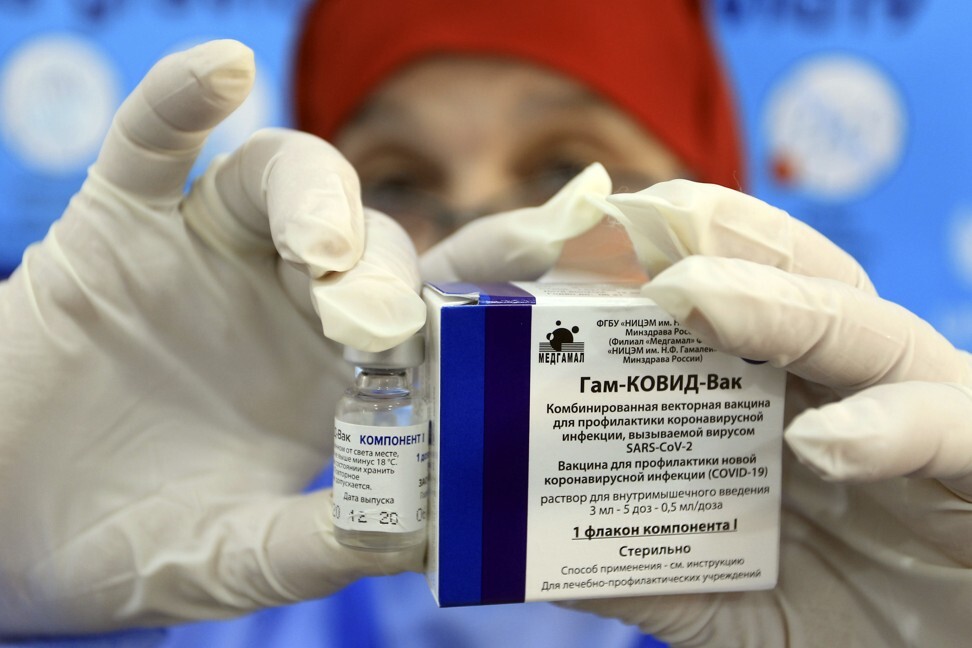
Coronavirus: Russia eyes German firm as co-partner in Sputnik V vaccine production
- Call to Germany’s IDT Biologika comes a day after The Lancet journal published trial results showing Sputnik V to be 91.6 per cent effective against Covid-19
- Sputnik V has been approved in more than 15 countries, including several ex-Soviet republics, but also Argentina, Tunisia and Pakistan
Russia has reached out to German biotechnology company IDT Biologika to explore jointly producing the Sputnik V coronavirus vaccine, the German health ministry said Wednesday.
It comes a day after The Lancet journal published trial results showing the Sputnik vaccine to be 91.6 per cent effective against Covid-19, defying international scepticism about the Russian-made jab.
A German health ministry spokeswoman said that Russia’s state-run Gamaleya research institute, which developed Sputnik V, and the Russian Direct Investment Fund (RDIF), which helped finance the project, “are interested in a co-partner for a possible production and have as such contacted IDT Biologika”.
“The contents or details of confidential discussions are not known,” she added. IDT, based in Dessau in eastern Germany, declined to comment when contacted by AFP.
Sputnik V has been approved in more than 15 countries, including several ex-Soviet republics but also Argentina, Tunisia and Pakistan.
In a call with reporters on Wednesday, Kremlin spokesman Dmitry Peskov said The Lancet article demonstrated the “reliability and effectiveness of the Russian vaccine”.
“And besides that, [it] probably also confirmed the justification of the accelerated registration of this vaccine in the Russian Federation”, he said, adding that the jab’s safety was “proven”.
The positive data from the Sputnik trial comes as the European Union (EU) faces growing impatience over its vaccination roll-out, which has been dogged by delivery delays and supply shortages.
AstraZeneca ‘to increase EU vaccine deliveries by 30 per cent’
German Chancellor Angela Merkel on Tuesday said “all vaccines” are welcome in the EU so long as they have been approved by the European Medicines Agency (EMA).
Merkel already spoke with Russian President Vladimir Putin last month about the Sputnik jab, offering the help of Germany’s Paul Ehrlich Institute in assisting Russia with the application process for the EMA.
“And if this vaccine is approved by the EMA, then we can talk about joint production or also about usage,” Merkel said at the time.
09:50
SCMP Explains: What's the difference between the major Covid-19 vaccines?
In France, an official in Emmanuel Macron’s office, who asked not to be named in line with protocol, said the French president and Merkel were aligned in welcoming any inoculations that met EU standards, and that geopolitics played no role.
The comment came in an answer to a question about whether Macron would consider purchasing Russia’s Sputnik V vaccine. At the same time, the French official noted that the Russian vaccine had not yet been approved by bloc.
France is taking on the lead role in defending Europe’s faltering vaccine programme, and in doing so, junior minister for EU affairs, Clement Beaune, has been especially vocal in criticising the United Kingdom’s decision to focus on administering the first dose and to rely on the AstraZeneca-University of Oxford vaccination, despite doubts over its efficiency on older people.
Russia moves a step ahead of China with Covid-19 vaccine data release
France’s health authority on Tuesday decided not to recommend giving the AstraZeneca shot to people over the age of 65. French scepticism stems from genuine concern, the official stressed, and was not down to a public relations war or anything else to do with politics.
The press briefing came after Macron met with representatives from local drug companies on Tuesday evening to encourage them to help produce coronavirus vaccines to boost supplies and end bottlenecks that have triggered shortages across the EU.
Production of the Moderna vaccine is set to begin in March in France, while the manufacturing of the Pfizer-BioNTech jab should start in April, Industry Minister Agnes Pannier-Runacher said in an interview with RTL radio earlier on Wednesday. Both vaccines have already been approved by the EU.

.png?itok=arIb17P0)