US approves pill fitted with digital sensor that tells doctors when you’re taking it
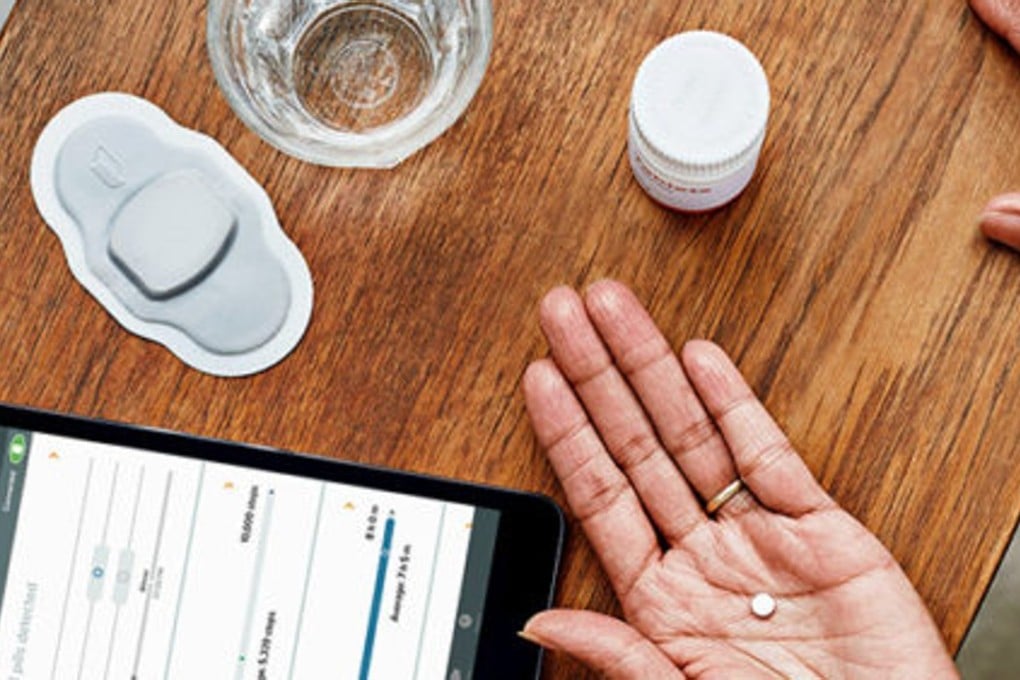
US regulators have approved the first pill that contains a digital tracking sensor to alert doctors and carers as to whether a patient is taking the medication as scheduled.
The pill, called Abilify MyCite (aripiprazole tablets with sensor), is designed for patients with schizophrenia, bipolar disorder and depression, according to the US Food and Drug Administration.
A patient ingests the pill, and a sensor inside the pill activates when it reaches the stomach fluids, sending a message to a wearable patch.
This patch then sends the information to a mobile app, so that a doctor and up to four carers, friends or family members can see the information on a website.
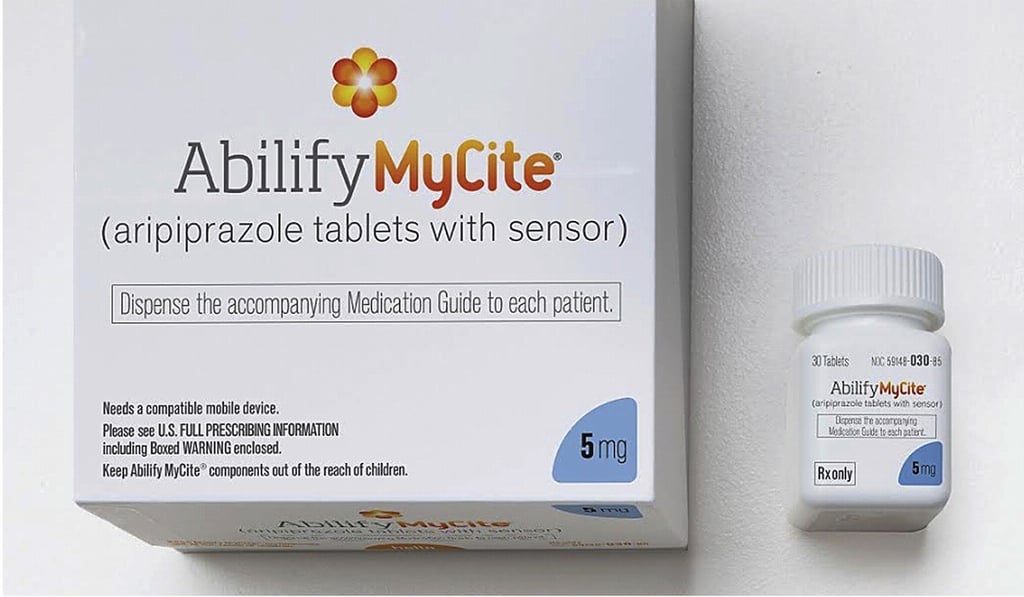
“Being able to track ingestion of medications prescribed for mental illness may be useful for some patients,” said Mitchell Mathis, director of the division of psychiatry products in the FDA’s Centre for Drug Evaluation and Research, in a statement Monday afternoon. “The FDA supports the development and use of new technology in prescription drugs and is committed to working with companies to understand how technology might benefit patients and prescribers.”