Advertisement
Moderna coronavirus vaccine safe, induces immune response, early results show
- No serious side effects among volunteers tested, but more than half reported mild or moderate reactions such as fatigue, headache, chills or muscle aches
- US company was first to start human testing of vaccine candidate on March 16. It will start phase 3 trials with 30,000 volunteers on July 27
Reading Time:3 minutes
Why you can trust SCMP
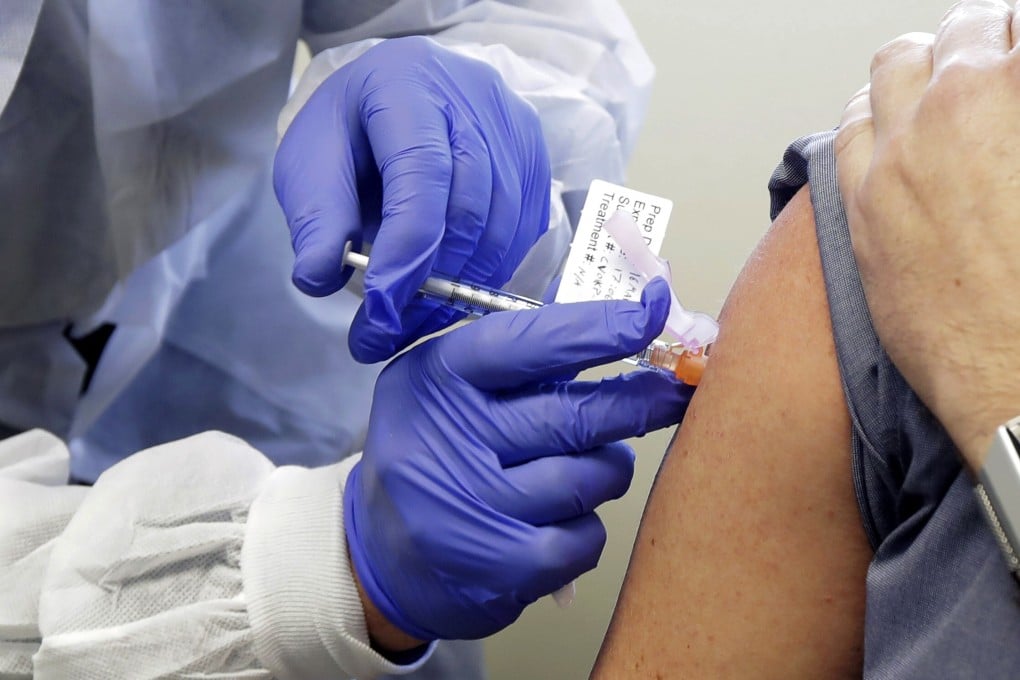
Moderna Inc’s experimental vaccine for Covid-19 was shown to be safe and provoked immune responses in all 45 healthy volunteers in an ongoing early-stage study, US researchers reported on Tuesday.
No study volunteers experienced a serious side effect, but more than half reported mild or moderate reactions such as fatigue, headache, chills, muscle aches or pain at the injection site.
These were more likely to occur after the second dose and in people who got the highest dose, the team reported in the New England Journal of Medicine.
Advertisement
Moderna shares jumped more than 15 per cent in after-hours trading on Tuesday.

Advertisement
Anthony Fauci, director of the US National Institute of Allergy and Infectious Diseases, whose researchers developed Moderna’s vaccine candidate, called the results “good news”, noting that the study found no serious adverse events and the vaccine produced “reasonably high” levels of virus-killing or neutralising antibodies.
Advertisement
Select Voice
Choose your listening speed
Get through articles 2x faster
1.25x
250 WPM
Slow
Average
Fast
1.25x