Pfizer says its coronavirus vaccine safely bolsters antibodies in younger children
- The results from a large-scale trial in kids ages five to 11 could pave the way for the inoculation of grade-school pupils
- Pressure to immunise children is on the rise in the US as a new school year begins amid a Delta-fuelled Covid-19 surge
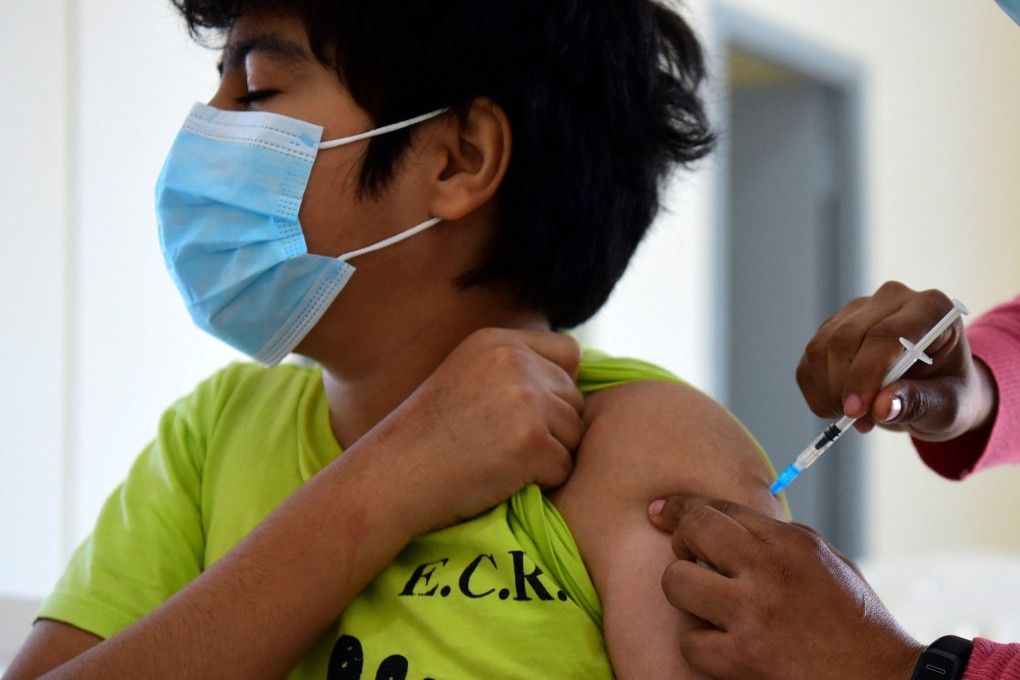
In a trial with 2,268 participants, two shots of a 10 microgram dose – one-third the adult shot – produced antibody levels comparable to those seen in a trial of 16-to-25-year-olds who got the adult dose, the companies said, with similar side effects.
Pfizer and BioNTech plan to begin a submission as early as this month for an emergency-use authorisation from the US Food and Drug Administration, a Pfizer spokesman said. The companies also plan to share the data with regulators in Europe.

02:34
Delta variant drives Covid-19 surge in southern US as unvaccinated are urged to get shots
A clearance would mark an important new phase of the immunisation campaign in the US, where the Pfizer vaccine already has full approval for people 16 and up and is authorised on an emergency basis for ages 12 to 15. And a paediatric clearance could arrive as millions of older Americans are receiving additional doses to bolster their initial shots.
The FDA is expected to decide whether to allow a booster shot for the Pfizer-BioNTech vaccine within days. On Friday, an advisory panel unanimously backed a third dose for people 65 and over, as well as those at high risk of severe complications, after voting against Pfizer’s request to authorise boosters for everyone 16 and older.