Advertisement
US FDA advisers back Pfizer Covid-19 vaccine for children ages 5 to 11
- The expert panel voted overwhelmingly to recommend the shots, saying the benefits outweigh the risks
- An authorisation for that group would be would be an important step toward reaching about 28 million children – most of them back in school – for inoculation
Reading Time:2 minutes
Why you can trust SCMP
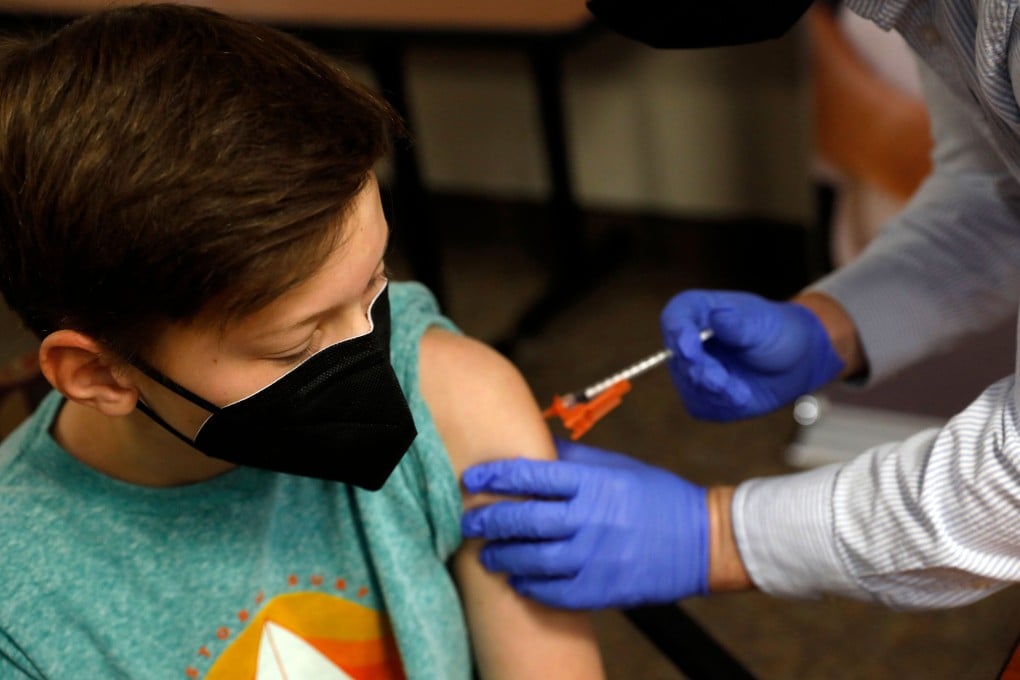
An expert panel on Tuesday voted overwhelmingly to recommend the US Food and Drug Administration authorise the Pfizer Inc and BioNTech SE Covid-19 vaccine for children ages five to 11, saying the benefits of inoculation outweigh the risks.
An authorisation for that age group would be would be an important regulatory step toward reaching about 28 million children for inoculation, most of them back in school for in-person learning.
The vaccine could be available to the younger age group as soon as next week. The FDA is not obliged to follow the advice of its outside experts, but usually does.
Advertisement
If the FDA authorises the shots for this age group, an advisory panel to the US Centres for Disease Control and Prevention will meet next week to make a recommendation on the administration of the vaccine. The CDC director will make the final call.

Advertisement
The companies have said their vaccine showed 90.7 per cent efficacy against the coronavirus in a clinical trial of children aged five to 11.
Advertisement
Select Voice
Select Speed
1.00x