Advertisement
US FDA authorises Pfizer’s coronavirus vaccine for young children
- The decision is expected to make the vaccine available to 28 million American children aged five to 11 years
- Only a few other countries, including China, Cuba and the United Arab Emirates, have cleared Covid-19 shots for children in this age group and younger
Reading Time:2 minutes
Why you can trust SCMP
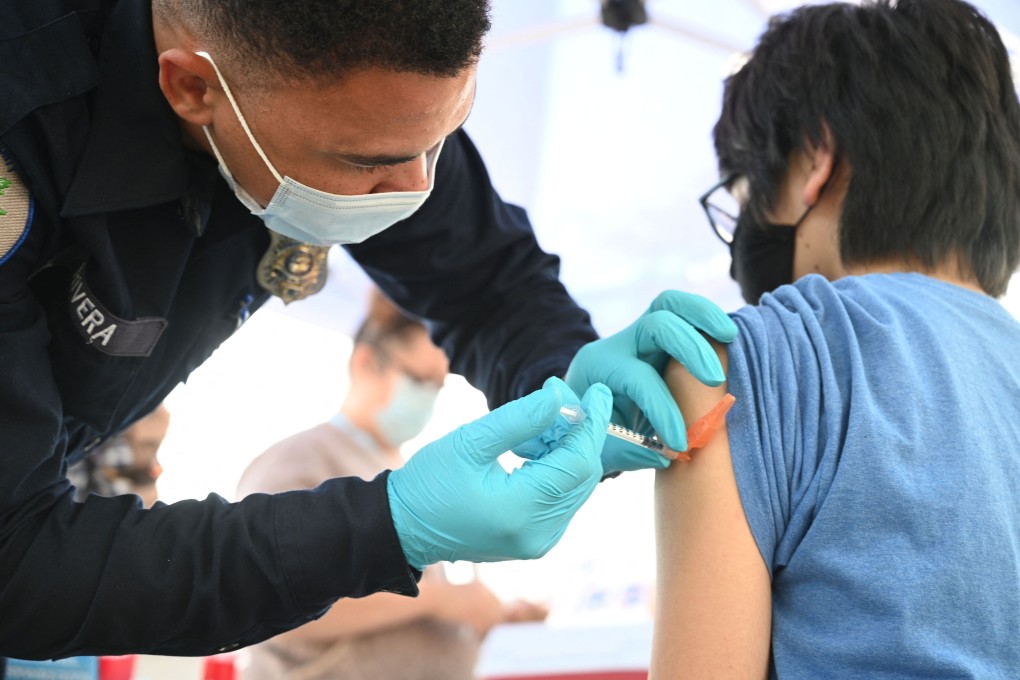
The US health regulator on Friday authorised the Pfizer Inc and BioNTech SE coronavirus vaccine for children aged five to 11 years, making it the first Covid-19 shot for young children in the United States.
The decision is expected to make the vaccine available to 28 million American children, many of whom are back in school for in-person learning.
It comes after a panel of advisers to the Food and Drug Administration (FDA) voted overwhelmingly to recommend the authorisation on Tuesday.
Advertisement
Only a few other countries, including China, Cuba and the United Arab Emirates, have so far cleared Covid-19 vaccines for children in this age group and younger.
Advertisement
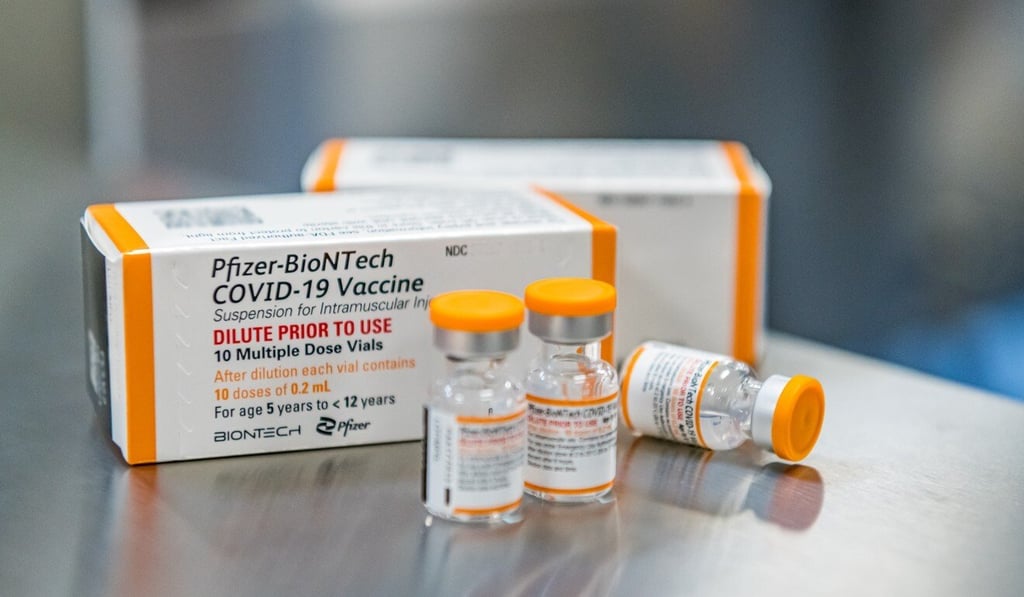
The FDA authorised a 10-microgram dose of Pfizer’s vaccine in young children, lower than the 30 micrograms in the original vaccine for those age 12 and older.
Advisers on the FDA panel said a lower dose could help mitigate some of the rare side effects.
Advertisement
Select Voice
Select Speed
1.00x