Hong Kong health department issues recall for five heart drugs containing valsartan that was made in China
An estimated 30,000 people could be affected by recall of pharmaceuticals used to treat hypertension and heart failure
Hong Kong’s Department of Health on Friday recalled five prescription drugs that are used to treat heart disease because they have the potential to cause cancer.
An estimated 30,000 people could be affected by the recall of the valsartan-containing drugs, which are prescribed to treat hypertension and heart failure.
The health department instructed wholesalers Actavis Hong Kong Limited and Hong Kong Medical Supplies Limited to recall the medicine from the market after an impurity – N-nitrosodimethylamine (NDMA) – was found in the valsartan, which was produced by a company in mainland China.
NDMA is classified as a probable carcinogen in humans.
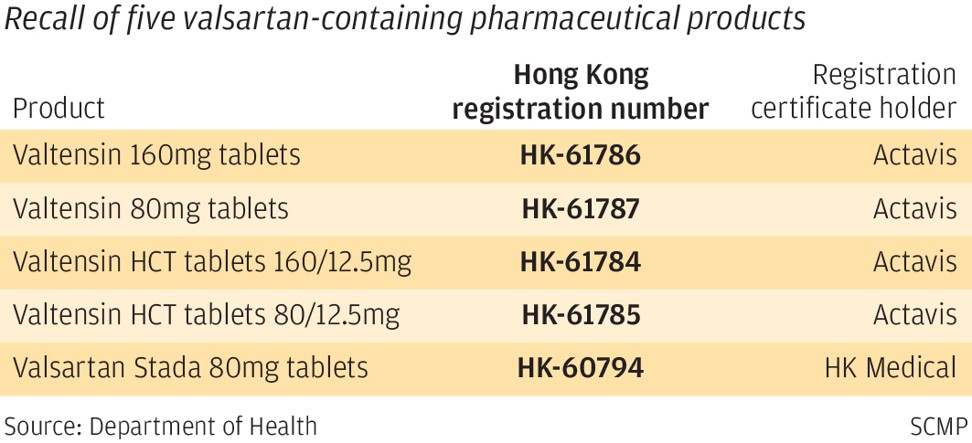
The affected products are Valtensin 160mg tablets, Valtensin 80mg tablets, Valtensin HCT tablets 160/12.5mg, Valtensin HCT tablets 80/12.5mg, and Valsartan Stada 80mg tablets. Four drugs were registered by Actavis with one registered by Hong Kong Medical Supplies.
Actavis HK and HK Medical Supplies confirmed the products registered in Hong Kong contained the affected material. According to the two wholesalers, the affected products have been supplied to private doctors and pharmacies. The Valtensin 80mg and 160mg tablets have also been supplied to the Hospital Authority.
The Hospital Authority said all public hospitals had been notified to stop prescribing the drugs.
“There are currently around 30,000 patients in public hospitals being dispensed with the anti-hypertensive drug concerned. Their condition will be reviewed by the attending doctor when they attend their next follow-up consultation at the clinic,” a spokesperson said.
The health department said patients should continue taking the drug, but should consult their doctor.
William Chui Chun-ming, president of the Society of Hospital Pharmacists of Hong Kong, agreed, saying the authority was still investigating the amount of contamination to “balance the risk and benefit”.
“The medicines in question are commonly used in the public [medical] sector for high blood pressure and heart failure, which means sudden cease of consumption may lead to fatal implications because uncontrolled high blood pressure can result in stroke, renal failure and acute vascular diseases,” Chui said.
Cardiologist Dr Bernard Wong Bun-lap said patients should consult their doctors as soon as possible and seek to changing medicine.
“The chance of contracting cancer is related the amount of carcinogen, as well as the patients’ physical condition. Patients are advised to use other medicines under doctors’ instructions no matter how much NDMA it takes to give them cancer,” Wong said.
Wong added that the medicine in question have been widely used in public hospitals because they were cheaper versions of the drug.
“One pill from the original factory can cost HK$6 [US$0.75] or more than HK$10, while those produced by other factories – mostly in developing countries – can be priced under HK$1 each,” Wong said.
Both companies have set up hotlines (Actavis: 3188 4288; HK Medical: 2806 3112) to answer related inquiries.