02:24
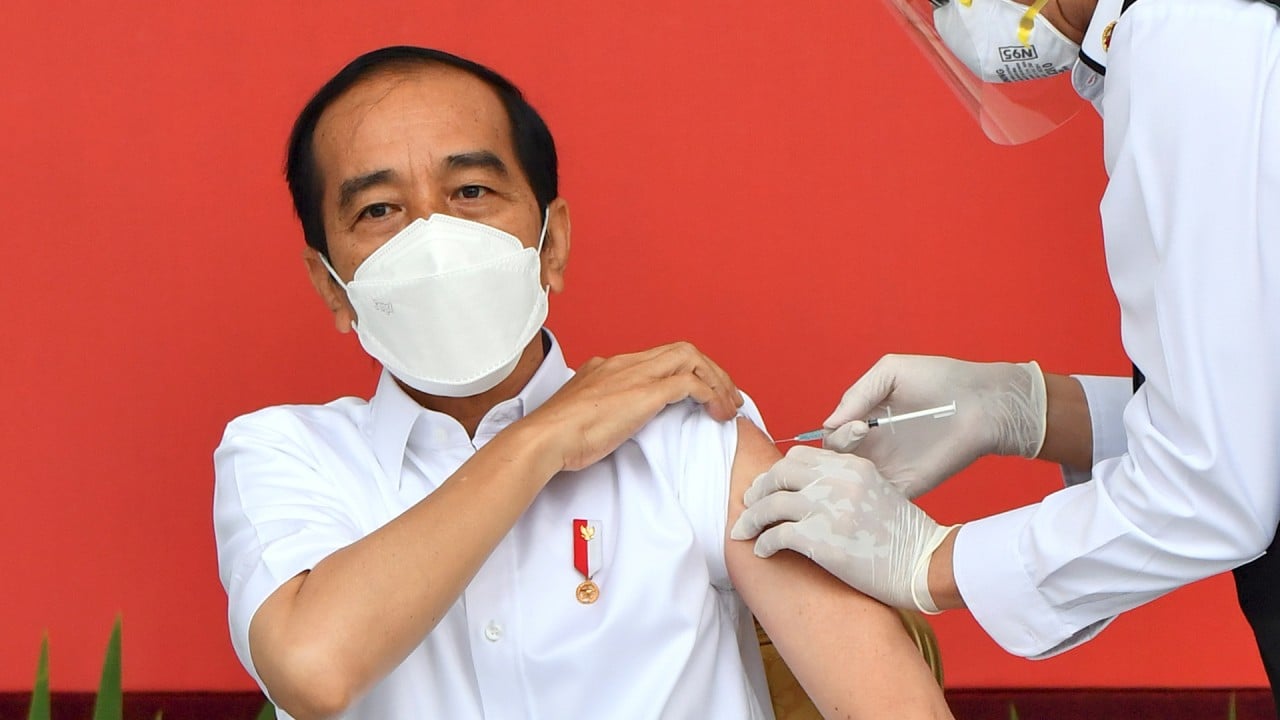
Joko Widodo gets first Sinovac vaccine shot as Indonesia starts mass Covid-19 inoculations

Indonesia’s ambitious vaccination drive to inoculate two thirds of its population of 270 million began on Wednesday when President Joko Widodo received a shot of Sinovac Biotech’s CoronaVac vaccine.
Southeast Asia’s largest economy intends to vaccinate its entire working population over the next 15 months, in a desperate bid to stem a pandemic that has infected some 869,000 people and killed 25,000.
Its success will rely partly on Bio Farma, a 130-year-old state-owned vaccine manufacturer.
As Indonesian and Chinese ministers met last August on Hainan island, Sinovac and Bio Farma sealed two agreements under which Sinovac would provide enough vaccine for 155 million doses (most of it in a bulk consignment) and Bio Farma would produce the individual vials.
If this arrangement works, and the two firms meet Indonesia’s domestic demand, the country could go on to become Southeast Asia’s production hub for Chinese-developed vaccines.
“We are set to receive 144 million doses from Sinovac by the first quarter of this year, and the rest will be delivered in July, so this is our programme for the first six months, to produce these vaccines,” Bio Farma Chief Executive Honesti Basyir told the House of Representatives on Thursday.

02:24
Joko Widodo gets first Sinovac vaccine shot as Indonesia starts mass Covid-19 inoculations
Bio Farma could produce one million doses per day, he added. So far, the company has received three million doses of pre-packaged CoronaVac vaccines, and 15 million doses in bulk. The company’s annual production capacity is three billion doses.
Bio Farma had also set aside up to 2.5 trillion rupiah (US$177.7 million) in capital expenditure this year, in which 500 billion rupiah would be used to “support the implementation of Bio Farma as a regional [vaccine production] hub,” Honesti told CNBC Indonesia on December 30.
However, Bio Farma must also meet its commitments to producing a share of the global Covid-19 vaccine supply. In October, the firm was appointed as one of the vaccine manufacturers for the Coalition for Epidemic Preparedness Innovations, or CEPI. It has agreed to produce up to 100 million vaccine doses by the fourth quarter of this year or first quarter of 2022, a feat it says is well within its capabilities.
“We have annual production capacity of at least 2.5 billion vaccine doses, and we have enough cold room to store them all, if what we have to produce is around 100 million doses, then it’s nothing compared to our annual production capacity,” said Bambang Heriyanto, corporate secretary at Bio Farma.
02:04
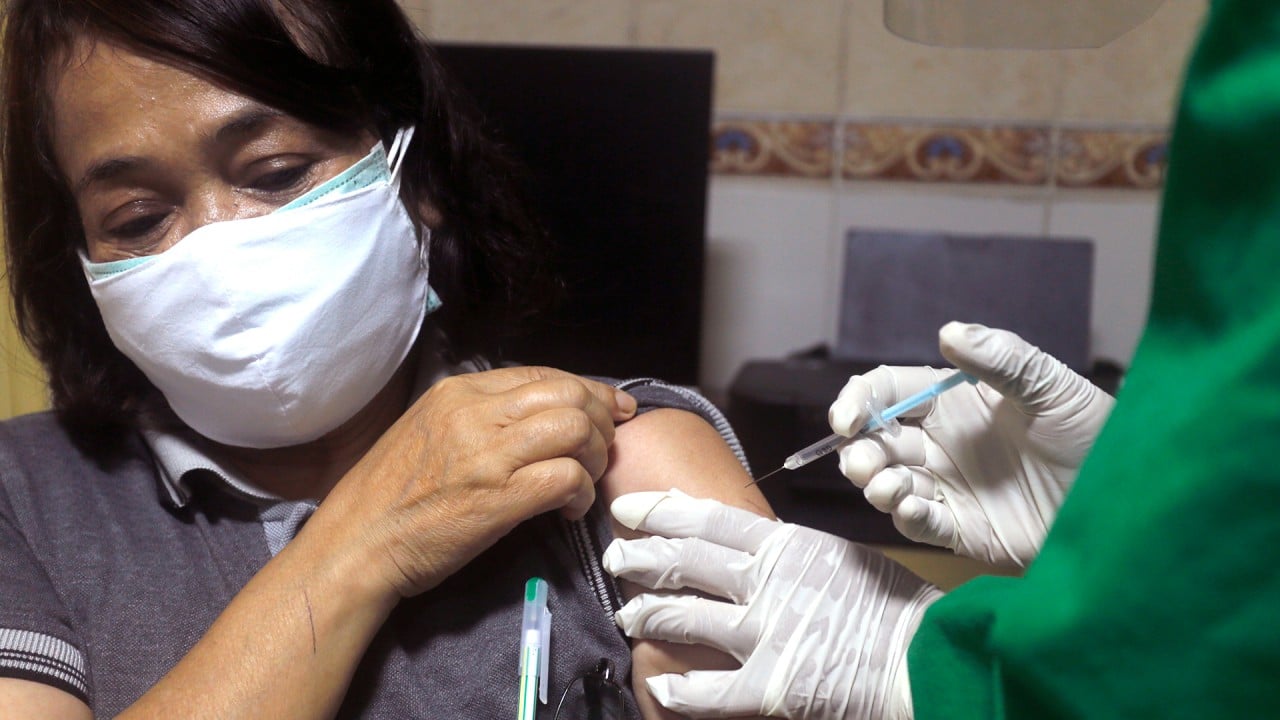
Indonesia says CoronaVac is 65.3% effective as Chinese-made Covid-19 vaccine makes inroads in Asia
WORLD CLASS?
While its name may be unfamiliar to foreign ears, Bio Farma has long been recognised by the World Health Organization for producing 16 vaccines for diseases including polio, diphtheria, tetanus, hepatitis B, and whooping cough. Its vaccines have been used in 150 countries, and it played a role in eradicating polio by developing the Novel Oral Polio Vaccine type 2.
Bio Farma was established as Parc-vaccinogène during the Dutch colonial period in 1890. Its name has changed several times and it was known as Bandung Epidemics Institute in 1942, when the country was under Japanese occupation.
Following upheaval during Indonesia’s transition to independence in 1945, it moved its headquarters from Bandung, in West Java, to Yogyakarta until 1949, when it returned to Dutch-occupied Bandung and changed its name to Landskoepok Inrichling en Instituut Pasteur.
The institution was named ‘Pasteur’ in a nod to Louis Pasteur, the renowned French biologist who was also the father of vaccination.
Indonesia eventually nationalised the institution in 1955, and changed its name to Perusahaan Negara Pasteur, before tweaking it to Perusahaan Negara Bio Farma in 1961. Since 1997, it has officially been named PT Bio Farma and is headquartered on Pasteur Street in Bandung. It is now the holding company of three other state-owned pharma firms, two of which are publicly listed.
In 1997, Bio Farma earned WHO pre-qualification for 12 types of vaccines – meaning the vaccines were safe and effective. This made it one of 30 vaccine producers from 22 countries that could export to more than 140 nations. In 2000, it founded the Developing Countries Vaccine Manufacturers Network, an alliance of 41 vaccine manufacturers in 14 developing countries and territories, whose mission is to ensure consistent and sustainable supply of quality vaccines at affordable prices in developing nations.
While the company has proven capable of producing world-class vaccines, its production capacity trails those of Indian vaccine manufacturers, which make 60 per cent of the world’s vaccines. India’s biggest vaccine producer, the Serum Institute of India, has said it is turning out between 60 million and 70 million Covid-19 vaccine doses a month, much more than Bio Farma’s 30 million doses per month.
“Our mission is to contribute vaccines for the global community, we also aspire to be on the same level as the world’s Big 5 pharma companies, but we still need to work hard to achieve that,” Bambang said.
“Our annual capacity is big, but [the products] are still low value, compared to pharma companies in developed nations whose production volumes are small but their products are high-value. We also make vaccines for government programmes.”

02:13
Only 60 graves left for Indonesian Covid-19 victims as cemetery in capital Jakarta fills up
VACCINE SCEPTICISM
Last year, Bio Farma began a late-stage trial of the CoronaVac vaccine, saying its production of the vaccine was dependent on the trial’s success. But scientists and health experts questioned why only 1,620 participants were involved, and why most participants were members of the general public rather than health care workers who would have higher exposure to the virus. In comparison, Brazil’s Phase 3 trial had 12,500 volunteers while Turkey’s had 7,300.
The trials have had a mixed outcome. The Indonesian trial that took place in the city of Bandung showed an efficacy rate of 65.3 per cent but the Brazilian one showed just 50.4 per cent. Sinovac said the design of the trials was not the same in all countries and that even so the results were sufficient to prove CoronaVac was safe and effective.
Achmad Utomo, a molecular biology expert and former researcher at the Jakarta-based Stem Cell and Cancer Institute, said it was peculiar that with “trillions of rupiah” budgeted for the trial, only 1,620 people were involved.
The small number of volunteers gave the impression that the clinical trial was inadequate, which “makes me doubt the future of science in Indonesia,” he said.
In response, Bio Farma said that the clinical trials only required 1,620 volunteers based on “calculation of total sample formula to attain immunogenicity and efficacy data”, and that the Bandung trial offered “supplementary data to third phase clinical trials [of the Sinovac vaccine] in other countries.”
Dicky Budiman, an epidemiologist at Australia’s Griffith University, defended the decision by Indonesia’s food and drugs agency to authorise CoronaVac for emergency use, pointing out that the Bandung trial data was based on how many volunteers had contracted the virus three months after receiving the second dose of the vaccine.
“The efficacy rate of 65.3 per cent is an adequate and strong base to say that Sinovac’s vaccine is safe and halal (permissible for use by Muslims),” he said.
Still, convincing the public to accept the vaccine will be a challenge for Indonesian authorities.
The country is expected to spend 20.9 trillion rupiah (US$1.49 billion) on procuring Sinovac’s Covid-19 vaccine and the country’s mass vaccination campaign could cost 66 million to 75 trillion rupiah (US$4.7 billion to US$5.34 billion).
A survey of 5,852 Indonesians by the WHO in October found only 57 per cent were willing to be inoculated. A survey by Bio Farma in the same month found 7.6 per cent of respondents were not willing to be inoculated, citing doubts about vaccine safety, while 27.6 per cent were undecided.