Advertisement
How long will a coronavirus vaccine take? A Q&A with Jerome Kim, head of the International Vaccine Institute
- It normally takes five to 10 years for a vaccine to be approved for market use, but during a pandemic, this timeline may be compressed, says Dr Jerome Kim
- From Britain to South Korea, firms and institutions are working on a vaccine for the Covid-19 disease, offering hope that more than one may be developed
Reading Time:6 minutes
Why you can trust SCMP
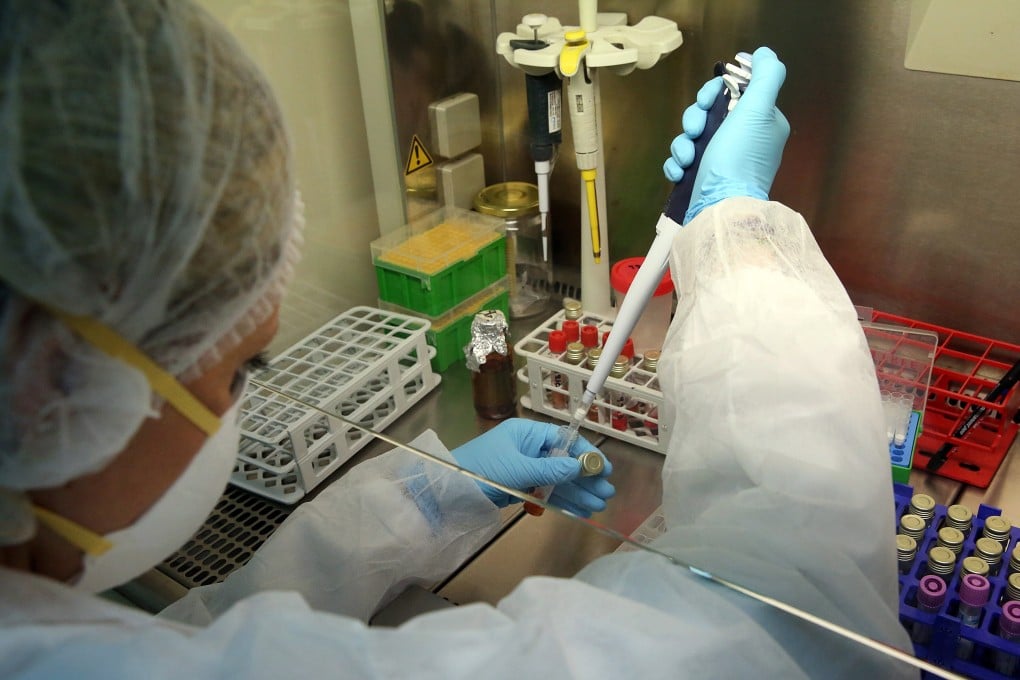
As the novel coronavirus pandemic continues to sicken thousands, universities and companies around the world are working on a vaccine for the Covid-19 disease.
Given the gravity of the crisis, the mission comes with obvious challenges – not least the lack of information on Covid-19, including whether an infected person can build a natural immunity to a second infection.
Dr Jerome Kim, the director general of the International Vaccine Institute and an expert on the evaluation and development of vaccines, tells This Week in Asia about the global race to bring a vaccine to market.
Advertisement

How close are we to a Covid-19 vaccine?
On average, it takes five to 10 years and costs US$1 billion to US$2 billion for a vaccine to reach the final approval to enter the market. There’s a failure rate of over 90 per cent, which highlights how challenging vaccine development is, even for large multinational firms.
Advertisement
But during a pandemic, this timeline may be compressed. After the first coronavirus outbreak was announced, it took only 2½ months for scientists to perform the first human test of a vaccine.
Advertisement
Select Voice
Select Speed
1.00x