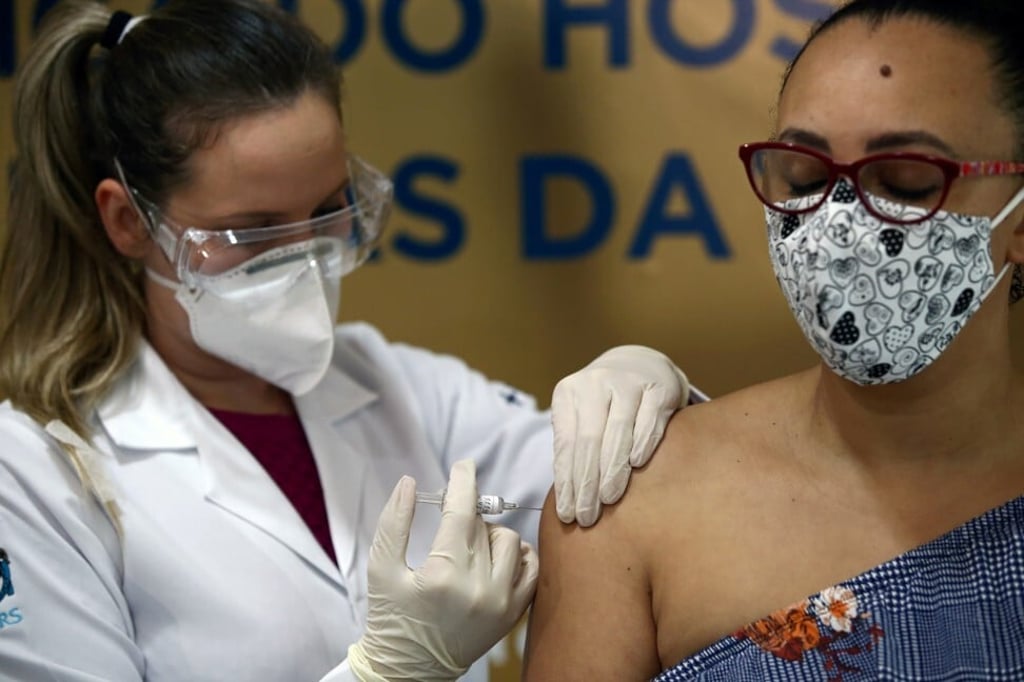
Indonesia’s Sinovac Covid-19 vaccine trial to continue, as Brazil halts theirs
- Brazil on Monday suspended a late-stage trial for the Chinese vaccine after a person reportedly died, though not because of the vaccine
- While epidemiologists say suspensions in vaccine trials are ‘normal’, one has cautioned Indonesia to proceed with its Sinovac vaccine trial with extra care
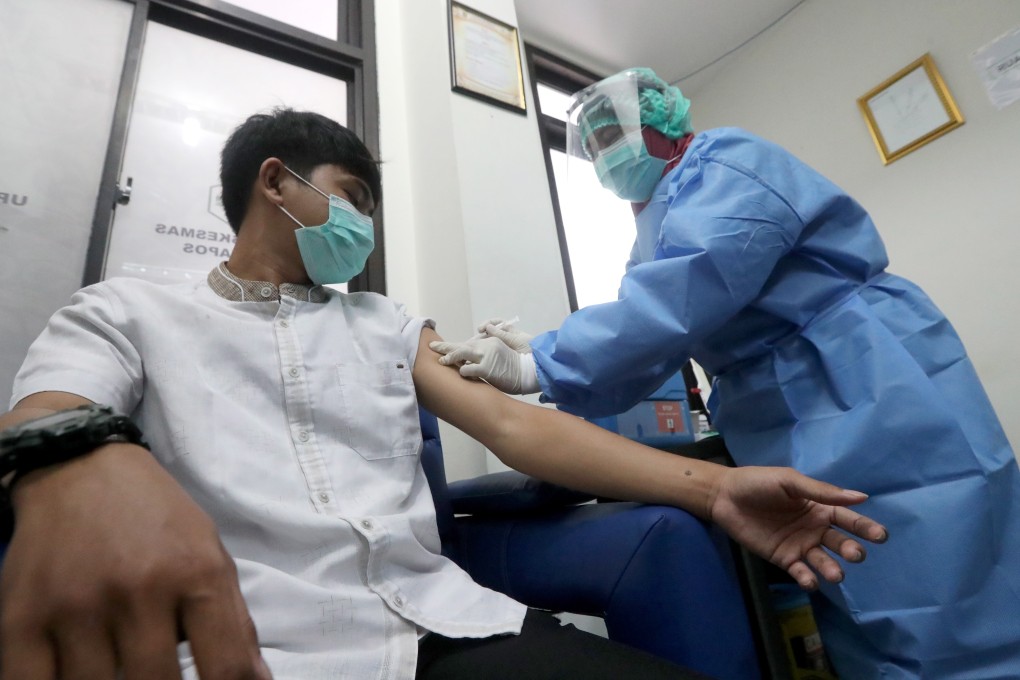
Edwin G. Pringadi, a spokesman for Bio Farma which teamed up with Sinovac to conduct trials and eventually produce 16 to 17 million doses of the vaccine, said the trial, with 1,620 volunteers in the province of West Java, was “going smoothly”.
The Brazilian trial involved more than 10,000 volunteers and was the first of Sinovac’s large late-stage trials to get under way. Turkey, Chile and Bangladesh are also conducting Sinovac vaccine trials.
Dimas Covas, the head of Sao Paulo’s medical research institute Butantan which is conducting the trial, said the decision was related to a death. He found the regulator’s announcement strange “because it’s a death unrelated to the vaccine”, Covas told local broadcaster TV Cultura, as quoted by Reuters.
Sinovac Biotech stood by the safety of its coronavirus vaccine, saying in a statement it was “confident in the safety of the vaccine”, and that the adverse incident was “unrelated to the vaccine”.