With Malaysia, UAE soon to make Chinese vaccines, does Beijing have an edge in vaccine diplomacy?
- The Sinovac and Sinopharm shots are in high demand from developing countries, even though scepticism about their efficacy persists
- China’s move to diversify production comes amid scrutiny of the AstraZeneca vaccine and as EU, India tighten vaccine exports
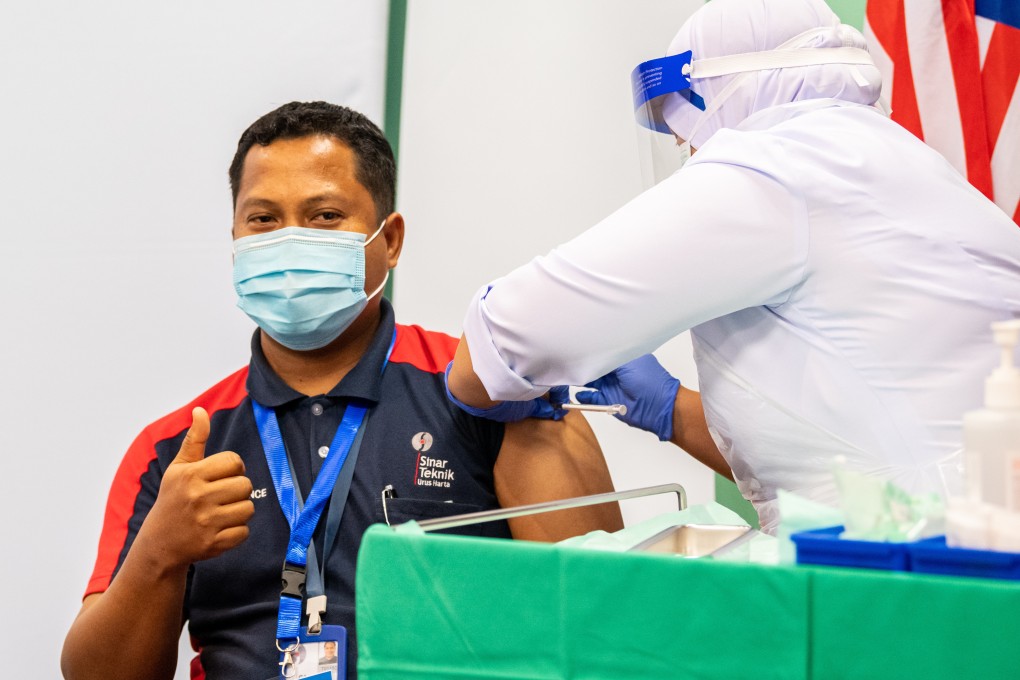
Indonesia’s Bio Farma has also signed a deal to manufacture the Sinovac jabs, which it currently imports from China and puts into vials. This is similar to Malaysian pharmaceutical group Pharmaniaga, which in January signed an agreement with Sinovac to purchase ready-to-fill vaccines, with plans to eventually manufacture them.
Chong Ja Ian, a political scientist and scholar of Chinese foreign policy at the National University of Singapore, said having production lines in the UAE and Indonesia brought Chinese vaccines closer to “potential users” in Africa, the Middle East, and Southeast Asia. “These conditions can clearly help with the implementation of any diplomatic efforts surrounding the supply of vaccines,” he said.
Sinopharm and Sinovac are among the five vaccines that have been granted regulatory approval by China’s drug regulator for domestic use. Currently, only a handful of countries – including the UAE, Turkey and Hungary – have approved the use of Sinopharm’s vaccine, while Sinovac is being used to inoculate the populations of Malaysia, Indonesia, Thailand and the Philippines, among others.