
What science says about CBD, derived from cannabis, used in food, drinks, lotions and cosmetics, and hailed as a cure-all – but banned in China
- The US Food and Drug Administration has not found adequate information showing how much CBD can be consumed, and for how long, before causing harm
- Nearly all CBD research has been done in rodents. As trials in humans grow, researchers expect it will be proven to be safe and effective
The first medical uses of cannabis date back to Emperor Shen Nung, the “father” of Chinese medicine. In the first Chinese pharmacopoeia, cannabis was recommended for fatigue, rheumatism and malaria, and its seeds were recommended to treat eczema, psoriasis and inflammatory diseases.
But cannabis was banned in many places in the 20th and 21st centuries because of its psychoactive properties. Its recent legalisation or decriminalisation in some jurisdictions has been accompanied by new lines of research focusing on the possible medical and therapeutic uses of its derivative cannabidiol, known as CBD, which does not possess these psychoactive properties.
The Hong Kong government banned CBD products from February 2023. In the United States, the Food and Drug Administration (FDA), citing safety concerns, has suggested that Congress develop a regulatory framework for them.

Many CBD product users are convinced they work, despite a lack of scientific evidence for manufacturers’ claims concerning their benefits.
We know next to nothing about breast milk. A new institute is fixing that
Here is what science has to say about CBD, its potential benefits and possible dangers.
Is CBD the same as cannabis, and can it get me high?
The compound CBD – cannabidiol – is one of the 113 cannabinoids found in cannabis plants. It has no psychoactive effects – that is, it won’t get you high. THC – tetrahydrocannabinol – is the psychoactive compound in cannabis that provides the intoxication.

If it doesn’t get me high, why is CBD banned?
Hong Kong CBD ban kicks in with more than 77,000 items surrendered
Is CBD safe?
Researchers generally feel that CBD is non-toxic and will be ultimately judged safe for most users.
It has shown few side effects and users’ anecdotal evidence suggests that CBD is safe.
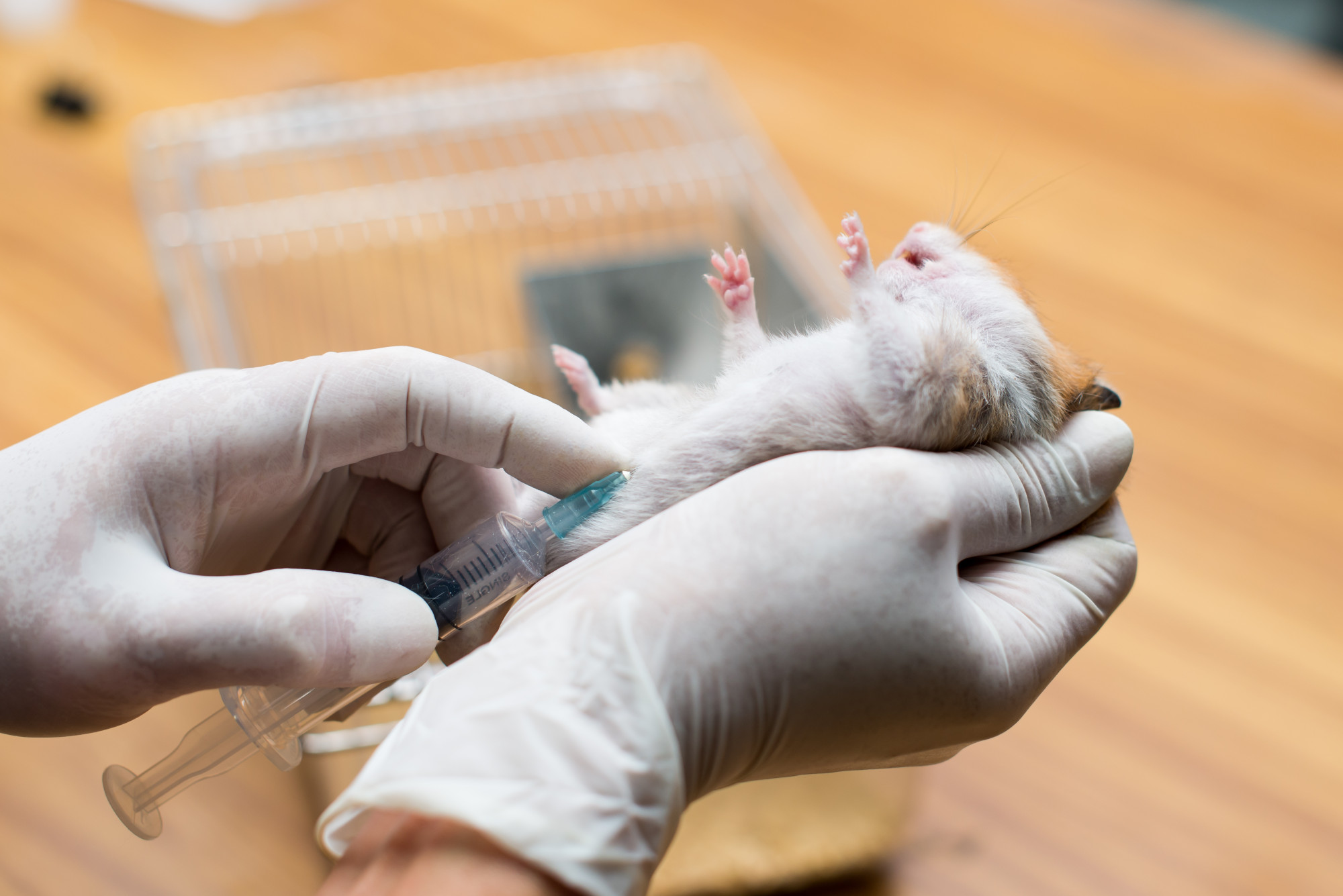
But CBD must be evaluated in clinical tests on humans. Nearly all research to date has been carried out on rodents, an established and useful scientific procedure. But what is safe for rodents may not be safe for humans.
“In vitro [laboratory] and preclinical studies [on rodents] have found CBD to be relatively low on toxicity,” write the authors of CBD: What Does The Science Say?, an academic compendium of all the scientific research about CBD to date, from the Massachusetts Institute of Technology Press.
The FDA has not found adequate information showing how much CBD can be consumed, and for how long, before causing harm
The FDA highlights other concerns regarding CBD use.
“The FDA has not found adequate information showing how much CBD can be consumed, and for how long, before causing harm. This is particularly true for vulnerable populations like children and those who are pregnant.”
So CBD has not been medically tested on human beings?
Testing of drugs takes place at three levels in the US: in vitro – in the lab at the cellular level; in vivo/preclinical, which generally takes place using rodents; and then three phases of clinical trials that take place in humans.
Most CBD studies have taken place in vitro, and in vivo using rodents.
Although 21 states have legalised its use, cannabis is still an illegal “Schedule One” drug in the US at the federal level, which causes CBD supply issues and has stymied research.
CBD: the benefits of cannabis compound, not appreciated by all
Has the FDA legalised CBD for some medical uses?
The FDA has approved one drug derived from CBD, Epidiolex, for use with two epileptic conditions in children: Dravet syndrome, which can appear in infants in their first year, and Lennox-Gastaut syndrome, at around the age of four.
It is also used to treat tuberous sclerosis complex, a rare genetic disease that causes non-cancerous tumours to grow in the brain and body.

Can CBD treat cancer?
Experiments in rodents have shown “the neuroprotective, tissue protective and even tumour-reducing effects of CBD”, say the authors of CBD: What Does The Science Say? It’s surprising that little of this positive research has been followed by human clinical trials, they note.
In tests on mice, CBD does not harm non-cancerous cells, but it does have a role in helping to kill cancer cells.
Hong Kong study finds more than threefold rise in drug-related internet posts
Can off-the-shelf CBD products control pain, as their makers claim?
Paracetamol, aspirin and ibuprofen can all have side effects, and opioids are addictive. Anecdotal evidence suggests that CBD, which has few side effects, may help to control pain, but there are few published studies.
A retrospective observational study published in 2021 in the Journal of Cannabis Research noted: “Despite growing evidence and interest, no real-world data studies have yet investigated patients’ reports of CBD impact on symptom control in the common expression of pain, anxiety, depression and poor well-being.”
A lack of research into CBD means it’s best to err on the side of caution
It reviewed the effects of CBD-rich treatment on 279 participants at a network of clinics in Quebec, Canada – where cannabis, including CBD, is legal – over more than six months. The treatment “had a beneficial impact on pain, anxiety and depression symptoms as well as overall well-being only for patients with moderate to severe symptoms; however, no observed effect on mild symptoms”, it concluded.
“The results of this study contribute to addressing the myths and misinformation about CBD treatment and demand further investigation.”

Can CBD help people suffering from psychosis?
A recent clinical test suggested that it might help those suffering from a loss of contact with reality.
“Although it is still unclear exactly how CBD works, it acts in a different way to antipsychotic medication, and thus could represent a new class of treatment [for psychosis],” said the study’s lead author, Professor Philip McGuire from King’s College London’s Institute of Psychiatry.
“Patients treated with CBD showed a significant reduction in symptoms, and their treating psychiatrists rated them as having improved overall.”

Many CBD products claim to cure acne, psoriasis and other skin conditions. Do they?
A 2022 report in the journal Pharmaceuticals (Basel), “Cannabis-Based Products for the Treatment of Skin Inflammatory Diseases: A Timely Review”, says CBD-based products have promising applications in skincare, in the cosmetics industry and for the topical treatment of skin diseases such as pruritus (itching), inflammatory diseases and even skin cancers.
Little research on the use of topical CBD has been performed on humans.
“The few available clinical trials are usually small, and lacking rigorous design … not providing enough data concerning safety and efficacy. Thus, there is a clear need for high-quality randomised controlled trials,” the report states.
What is the future of CBD products?
The Pharmaceuticals (Basel) report suggests that it is key to separate the use of cannabis for recreational purposes from its medical use.
It concludes: “It is likely that, as knowledge increases, there will be developments in the legal status of cannabis-based medicines, with more countries approving their use.”
