
In Modi’s India, political row erupts over home-grown coronavirus vaccine
- Knives are out for the Covaxin vaccine after it was granted emergency approval before final-stage human trials had been completed
- Squabbles between politicians from the BJP and other parties added to the simmering unrest among the Muslim and Hindu communities over the vaccine’s alleged contents
So it was on Sunday, when the drug regulator announced the approval of two vaccines for emergency use. One was Oxford-AstraZeneca’s Covishield, while the other was Covaxin from local pharmaceutical firm Bharat Biotech.
The knives were out for Covaxin just hours after the announcement, when it emerged that the home-grown vaccine was granted restricted approval even though final-stage, or phase-three, human trials had not been completed. Health experts have widely criticised the move as premature, pointing out that there is no publicly available data on its efficacy.
How will India vaccinate 1.3 billion people? Its army of auxiliary nurses and midwives know
Krishna Ella, the chairman and managing director of Bharat Biotech, on Monday defended Covaxin and said the firm was “confident” over its use. He said the company should finish final-phase recruitment within “two or three days” and that Covaxin would feature in two peer reviews in international health journals on January 10. A full readout for the vaccine’s phase-three efficacy data should come between March and October, according to a slide show he presented.

Bharat Biotech had already produced about 20 million doses, Ella said, which would be increased to about 150 million before July or August. The firm had previously said Covaxin – which uses a dead version of the virus – has efficacy rates of at least 60 per cent.
This reference set off alarm bells in the opposition Congress party, with MP Sashi Tharoor condemning the approval as “premature” and “dangerous”. Samajwadi party president Akhilesh Yadav called it a “BJP vaccine”, referring to Modi’s ruling Bharatiya Janata Party, and said that as such it could not be trusted.
Coronavirus vaccine: Asia-Pacific countries tread cautiously in roll-out
The political melee over the vaccine has added to already simmering unrest in the Muslim and Hindu communities. A few Muslim clerics have claimed that the Chinese vaccine contains pork gelatin, which is forbidden for Muslims, and called for the Indian government to reveal the contents of any vaccine that is going to be distributed. In the other camp, a Hindu spiritual leader thundered that if blood from cows, which the religion considers sacred, was used in any vaccine, they would veto it.
Trying to strike a sane note, India’s Drugs Controller General, V.G. Somani, has said Covaxin was safe and provided a good immune response. He said the vaccine, which was partly funded by the government, was given an emergency green light so the country had more options in case mutant strains emerged.
02:15
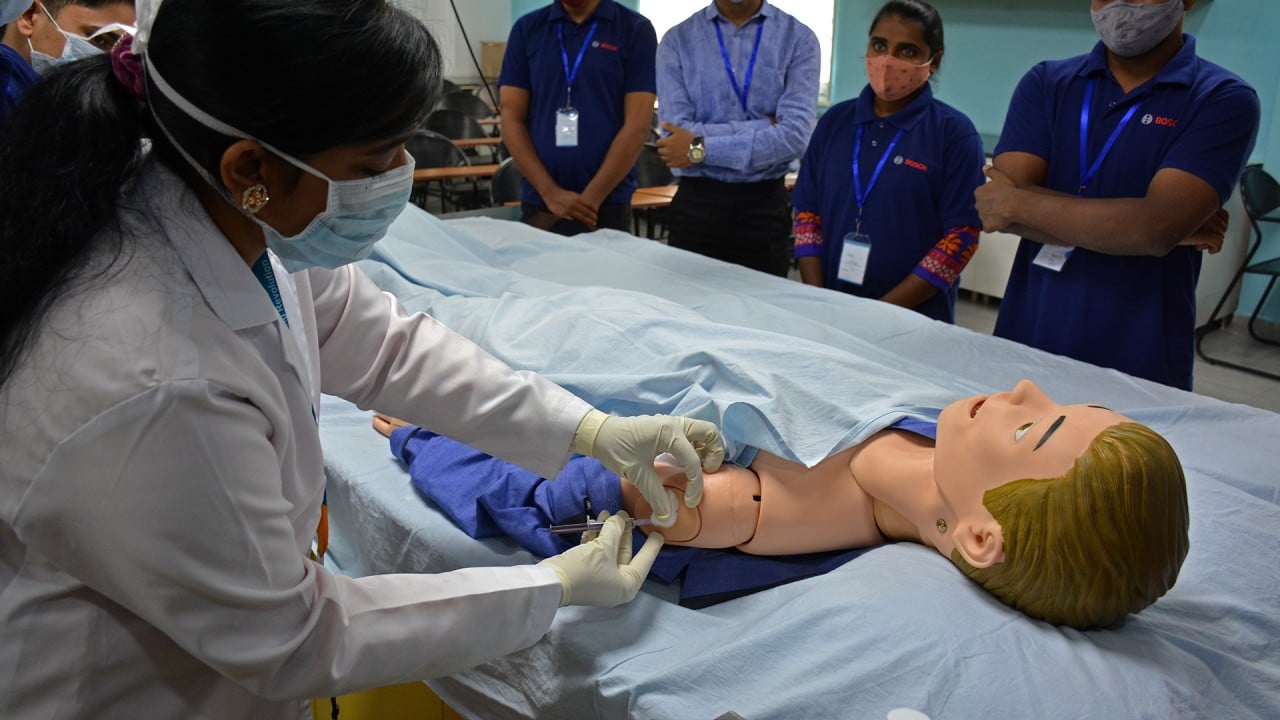
India trains workers to handle Covid-19 mass vaccination programme
BJP leaders, who had assumed the approval of Covaxin would be warmly received, seemed taken aback at the attacks – but hit back, accusing the Congress party of ridiculing Indian achievements.
“Time and again we have seen whenever India achieves something commendable, that will further public good, Congress comes up with wild theories to oppose and ridicule the accomplishments,” said BJP President J.P. Nadda.
Ella from Bharat Biotech had a similar reaction on Monday, saying the controversy showed that vaccine development in the country was “getting political”.
For India’s Mukesh Ambani, dethroned as Asia’s wealthiest man by China’s Zhong Shanshan, even humble pie tastes rich
“Why are Indian scientists being bashed?” he said, adding that no one in his family was a member of any party. “When Britain does something, [the reaction is] ‘Oh it’s great’ – I want Indian scientists to get recognition globally.”
More encouraging were the results of Saturday’s nationwide drill to see if India was prepared to embark upon its mammoth vaccination exercise, which saw teams in 125 districts across the country check to see there were sufficient fridges and syringes, stable electricity, and space for social distancing in vaccination centres.
The dry run went smoothly, but some glitches were identified – the internet was too slow to upload the data in some centres, while another worry was the delay that could arise if staff suddenly had to deal with someone who suffered serious side effects.
Additional reporting by Bloomberg

